Abstract
Objective
The use of direct lumbar interbody fusion (DLIF) has gradually increased; however, no studies have directly compared DLIF and transforaminal lumbar interbody fusion (TLIF). We compared DLIF and TLIF on the basis of clinical and radiological outcomes.
Methods
A retrospective review was performed on the medical records and radiographs of 98 and 81 patients who underwent TLIF and DLIF between January 2011 and December 2012. Clinical outcomes were compared with a visual analog scale (VAS) and the Oswestry disability index (ODI). The preoperative and postoperative disc heights, segmental sagittal/coronal angles, and lumbar lordosis were measured on radiographs. Fusion rates, operative time, estimated blood loss (EBL), length of hospital stay, and complications were assessed.
Results
DLIF was superior to TLIF regarding its ability to restore disc height, foraminal height, and coronal balance (p<0.001). As the extent of surgical level increased, DLIF displayed significant advantages over TLIF considering the operative time and EBL. However, fusion rates at 12 months post-operation were lower for DLIF (87.8%) than for TLIF (98.1%) (p=0.007). The changes of VAS and ODI between the TLIF and DLIF were not significantly different (p>0.05).
Conclusion
Both DLIF and TLIF are less invasive and thus good surgical options for treating degenerative lumber diseases. DLIF has higher potential in increasing neural foramina and correcting coronal balance, and involves a shorter operative time and reduced EBL, in comparison with TLIF. However, DLIF displayed a lower fusion rate than TLIF, and caused complications related to the transpsoas approach.
Go to : 
Various lumbar fusion approaches have been used to treat lumbar degenerative disc diseases, aiming at reducing low back pain, radiculopathy, and disability. Numerous comparative studies on the radiological and clinical outcomes of posterior lumbar interbody fusion (PLIF), transforaminal lumbar interbody fusion (TLIF), and anterior lumbar interbody fusion (ALIF) have been published13,21,25,31).
TLIF, which was first described by Harms and Rolinger9) has been widely used for its safety, good outcomes, and a high fusion rate, amongst a variety of lumbar interbody fusion approaches5,8,9,11). Surgeons are comfortable performing TLIF because it utilizes a posterior approach, and TLIF reduces dural retraction and enables direct neural decompression. However, this procedure carries risks for complications such as posterior spinal muscle injury, dural tears, and cerebrospinal fluid (CSF) leakage15,16,26).
Recently, McAfee et al.22) introduced direct lumbar interbody fusion (DLIF) by using a transpsoas retroperitoneal approach, and Ozgur et al.24) proposed minimally invasive DLIF via a tubular system. DLIF allows surgeons to avoid the shortcomings of PLIF and TLIF, such as posterior element injury and dural tears, as well as those of ALIF, such as retrograde ejaculation due to great vessel injury and hypogastric sympathetic plexus injury2,27,28). However, DLIF can lead to psoas muscle injury and various types of nerve damage4,10,20).
Until now, no comparative studies on the outcomes of DLIF and other fusion procedures based on radiological and clinical approaches have been conducted. The aim of our study was to analyze the outcomes of DLIF and TLIF considering the radiological outcomes, functional disability, pain, fusion rates, and patients' complications.
Go to : 
This single-center study was conducted by 2 senior surgeons between January 2011 and December 2012. TLIF and DLIF were performed by the surgeons to treat equivalent lumbar degenerative diseases including spinal stenosis, spondylolisthesis, and recurrent disc herniation. As DLIF is not suitable for diseases with L5-S1 involvement, patients with diseases involving the L5-S1 level were excluded from the study. For the 2 groups of patients, the age, extent of the operation, operative levels, and bone mineral density (BMD) were examined. The cage height used in each procedure was also assessed.
The TLIF group underwent unilateral open TLIF (fusion material : autologous bone), pedicle screw fixation on the surgical side, and contralateral pedicle screw fixation via an interfascial approach. The TLIF group was treated by using a single capstone cage (Medtronic, Minneapolis, MN, USA). The DLIF group underwent minimal invasive DLIF [fusion material : demineralized bone matrix (DBM)] by using a transpsoas retroperitoneal approach and percutaneous pedicle screw fixation. DLIF was performed using a DLIF system (Medtronic, Memphis, TN, USA) and a percutaneous pedicle screw fixation system (SEXTANT II® or Longitude system, Medtronic, Memphis, TN, USA). In addition, additional posterior decompression was performed for patients in the DLIF group (33 of 81 patients) who had severe central spinal stenosis or ruptured disc herniation. To compare patients with the same disease, patients who underwent posterior decompression in the DLIF group were included in the study (Fig. 1).
Pre- and post-operative clinical outcomes were compared between the groups by using the scores obtained with the visual analog scale (VAS) and Oswestry disability index (ODI). Additionally, we assessed the length of hospital stay, operative time, and estimated blood loss (EBL) for each group.
We measured the anterior and posterior disc heights, foraminal height, segmental sagittal and coronal angles, and lumbar lordosis on the preoperative and 12 months postoperative radiographs.
The measurements were performed by using plain lateral radiographs taken with the patients in the neutral position. The segmental sagittal/coronal angles were defined as the cobb angle of vertebral bodies adjacent to the operative level. Lumbar lordosis was defined as the angle between the upper endplates of L1 and S1 by using the Cobb method. Fusion was evaluated by using the Bridwell et al.3) fusion grade system. According to this system, fusion grades are defined as follows : 1) grade 1, complete remodeling with trabeculae across the disc space; 2) grade 2, intact graft with no lucent lines observed between the graft and the adjacent endplates; 3) grade 3, intact graft but a radiolucent line is present between the graft and an adjacent endplate; and 4) grade 4, lucency along an entire border of the graft or around a pedicle screw or the subsidence of the graft. On the basis of the classification system, we considered grades 1 and 2 as successful fusion. We assessed fusion rates at 12 months post-operation. The measurements and evaluations on the basis of the radiographs were initially performed by one surgeon, and the 2 other surgeons reviewed and confirmed the results.
We compared the radiological and clinical outcomes between the TLIF and DLIF groups by using an unpaired Student's t-test, Mann-Whitney U test, and the chi-squared test. We also compared the radiological and clinical outcomes pre- and post-surgery by using a paired t-test and Wilcoxon signed-rank test. Descriptive data were presented as mean±SD, and statistical significance was accepted as p<0.05.
Go to : 
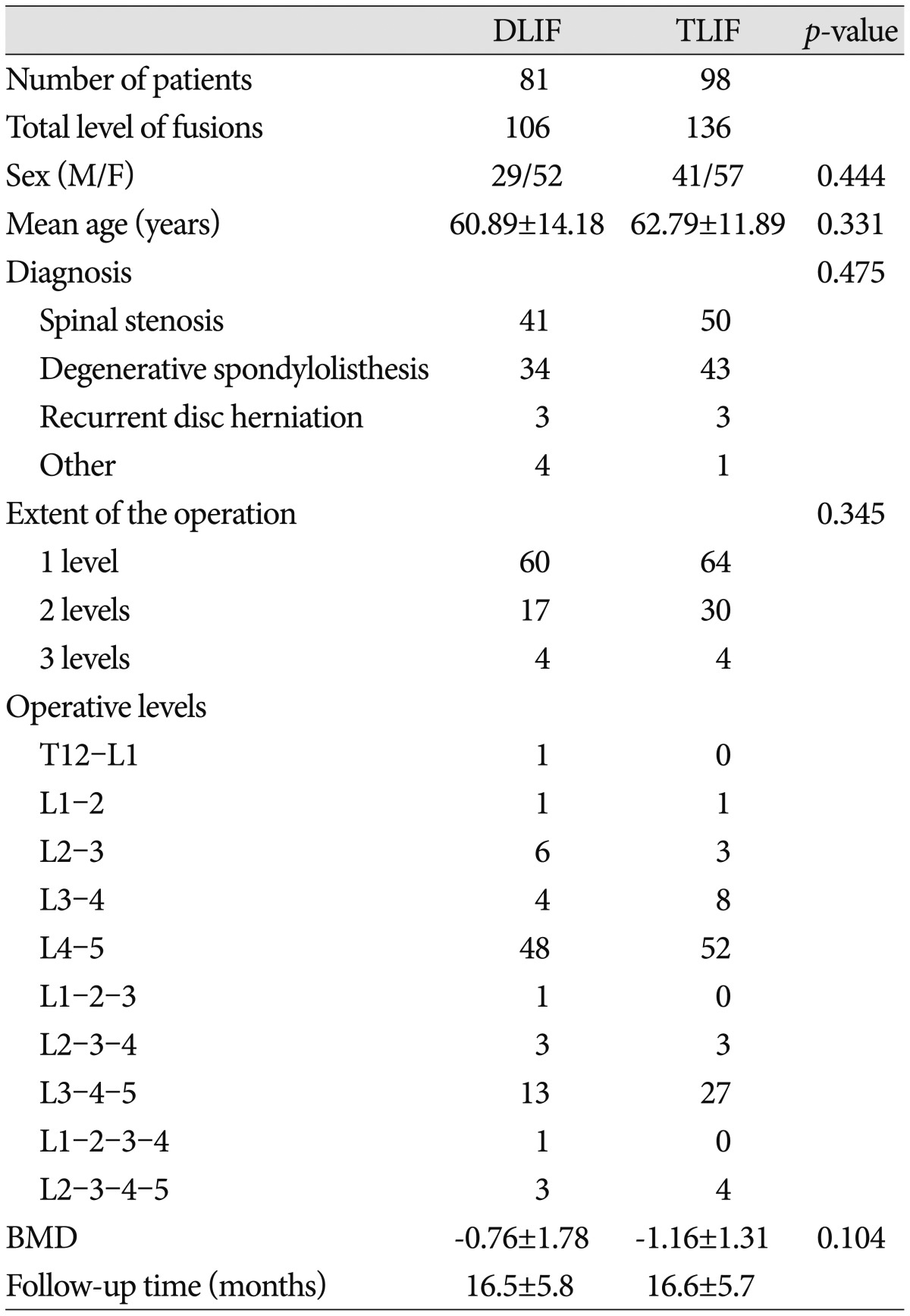
The study included 81 patients who underwent DLIF (106 levels; 29 men and 52 women) and 98 patients who underwent TLIF (136 levels; 41 men and 57 women) to treat nearly identical lumbar degenerative diseases.
The mean ages of the DLIF and TLIF groups were 60.89±14.18 years and 62.79±11.89 years, respectively. The mean BMD T-score was -0.76±1.78 in the DLIF group and -1.16±1.31 in the TLIF group. The preoperative diagnosis, extent of the operation, and operative levels are presented in Table 1. No statistically significant differences (p>0.05) were detected between the 2 groups in terms of the age, sex, preoperative diagnosis, extent of the operation, operative levels, and BMD (Table 1). The mean follow-up period was 16.5±5.8 months in the DLIF group and 16.6±5.7 months in the TLIF group. The most frequently used cage heights were 12 mm and 14 mm for both operative methods, with the cage heights being 10-16 mm in the DLIF group and 8-14 mm in the TLIF group. The mean cage heights were 12.94±1.30 mm and 12.45±1.59 mm in the DLIF and TLIF groups, respectively (p=0.043).
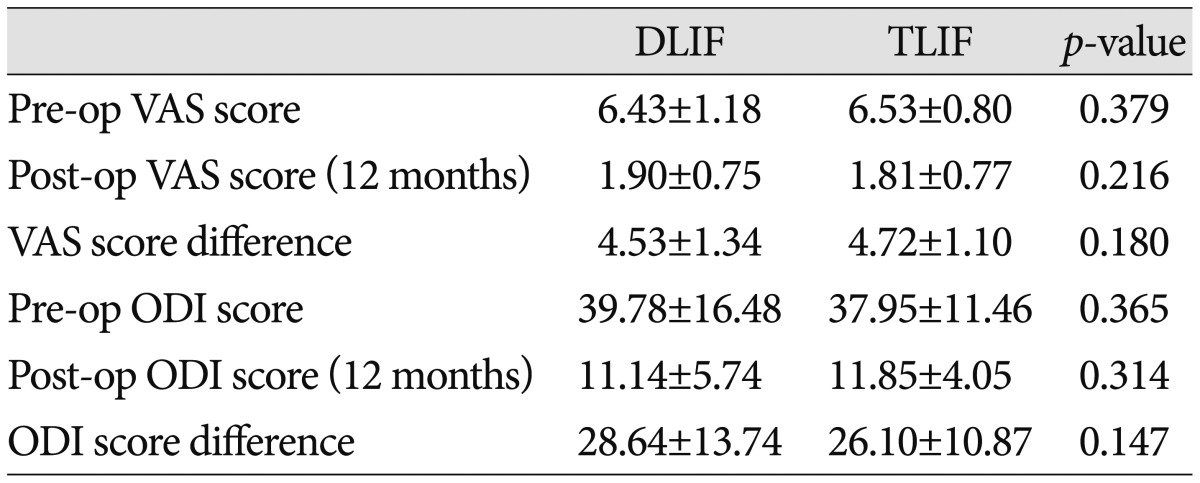
The preoperative VAS scores in the DLIF and TLIF groups were 6.43±1.18 and 6.53±0.80, respectively, whereas the corresponding post-operative VAS scores were 1.90±0.75 and 1.81±0.77, respectively. In both groups, the postoperative VAS scores revealed significant improvement in pain compared with the preoperative values (p<0.001). No between-group differences in the pre- and post-operative VAS scores were noted (p>0.05). In addition, the variation of the VAS scores pre- and post-surgery was not different between the groups (p=0.180). The preoperative ODI values in the DLIF and TLIF groups were 39.78±16.48% and 37.95±11.46%, respectively, versus postoperative values of 11.14±5.74% and 11.85±4.05%, respectively. In both groups, the postoperative ODI scores revealed significant improvements in pain compared to the preoperative values (p<0.001). No between-group differences were noted for the preoperative and postoperative ODI values (p>0.05). In addition, the variation of the ODI values pre- and post-surgery was not different between the groups (p=0.147) (Table 2).
The mean operative times were 128.83±33.23 min for 1-level surgery, 145.59±32.30 min for 2-level surgery, and 183.75±37.50 min for 3-level surgery in the DLIF group, versus 138.91±42.43 min, 194.67±36.76 min, and 252.50±22.54 min, respectively, in the TLIF group. A significant difference between the groups was observed for 2- and 3-level surgery (p<0.001) but not for 1-level surgery (p>0.05).
The mean EBL values were 153.83±104.75 mL, 173.53±79.92 mL, and 200.00±70.71 mL for 1-, 2-, and 3-level surgery, respectively, in the DLIF group, compared to 215.47±157.82 mL, 305.00±149.34 mL, and 462.50±165.90 mL, respectively, in the TLIF group. Significant differences between the groups in EBL were observed (p<0.05).
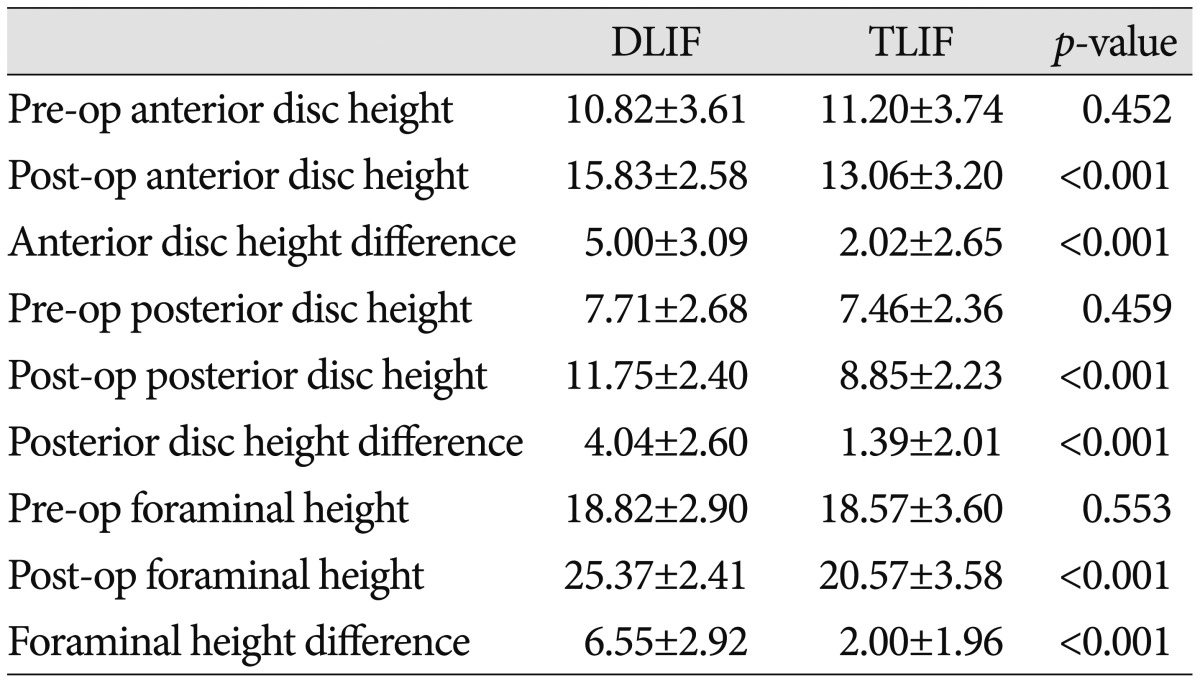
A summary of the radiographic outcomes is presented in Table 3, 4. The pre- and post-surgical anterior disc, posterior disc, and foraminal heights were not significantly different (p>0.05). However, the anterior disc height changed from 10.82±3.61 mm preoperatively to 15.83±2.58 mm postoperatively in the DLIF group, and from 11.20±3.74 mm preoperatively to 13.06±3.20 mm postoperatively in the TLIF group; both changes were statistically significant (p<0.001). The difference between the pre- and post-operative anterior disc height was greater in the DLIF group (5.00±3.09 mm vs. 2.02±2.65 mm; p<0.001).
In addition, the pre- and post-operative changes in the posterior disc height were significantly different in both groups. The pre- and post-operative posterior disc heights were 7.71±2.68 mm and 11.75±2.40 mm, respectively, in the DLIF group, compared to 7.46±2.36 mm and 8.85±2.23 mm, respectively, in the TLIF group (p<0.001). However, the change of the posterior disc height was greater in the DLIF group (4.04±2.60 mm vs. 1.39±2.01 mm; p<0.001) (Table 3).
The foraminal height changed from 18.82±2.90 mm preoperatively to 25.37±2.41 mm postoperatively in the DLIF group, and from 18.57±3.60 mm preoperatively to 20.57±3.58 mm postoperatively in the TLIF group (p<0.001). The change of the foraminal height was significantly greater in the DLIF group (6.55±2.92 mm vs. 2.00±1.96 mm; p<0.001) (Table 3).
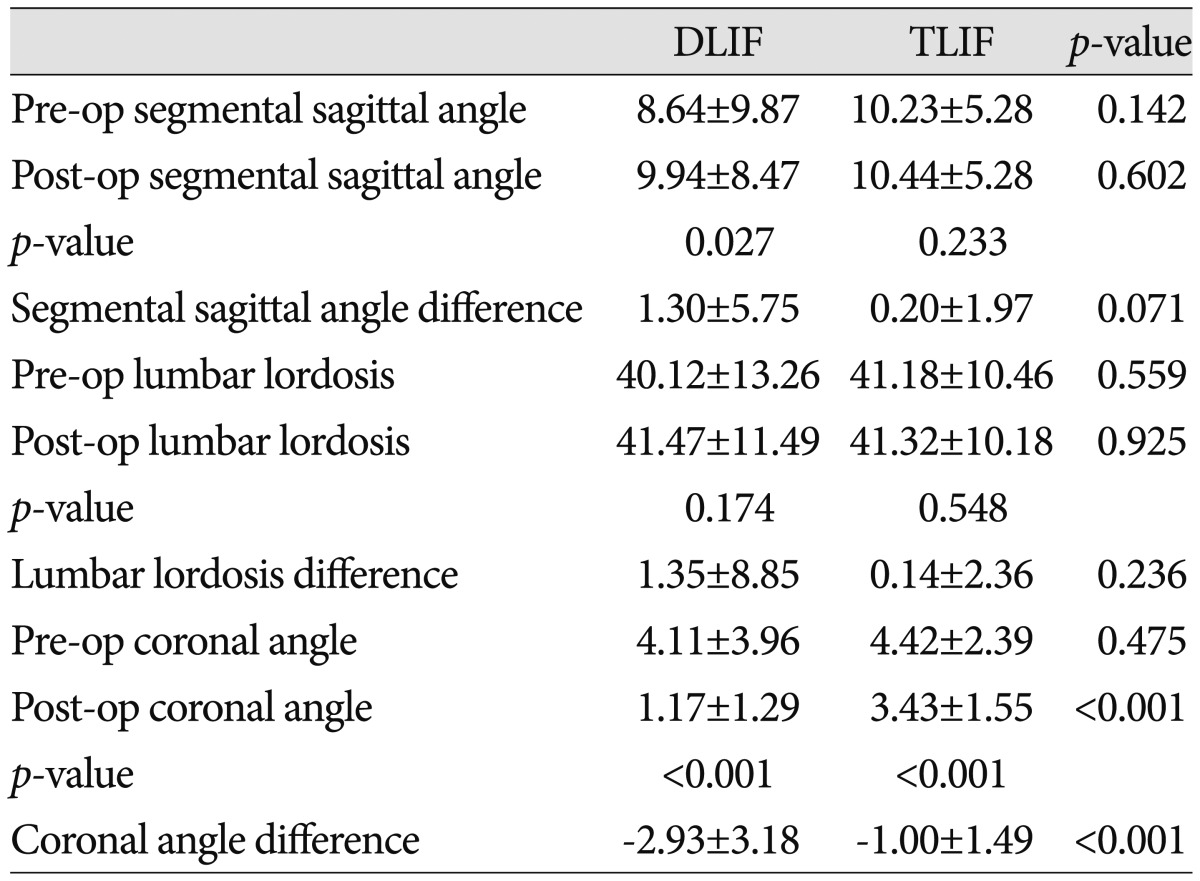
Prior to surgery, the segmental sagittal/coronal angles and lumbar lordosis were not different between the groups (p>0.05). In the DLIF group, the segmental coronal angle changed from 4.11± 3.96° preoperatively to 1.17±1.29° postoperatively, whereas the value changed from 4.42±2.39° preoperatively to 3.43±1.55° postoperatively in the TLIF group (p<0.001). The amount of variation in the segmental coronal angle was -2.93±3.18° in the DLIF group, compared to -1.00±1.49° in the TLIF group (p<0.001). The segmental sagittal angle significantly increased from 8.64±9.87° preoperatively to 9.94±8.47° postoperatively in the DLIF group (p=0.027). However, lumbar lordosis did not change significantly post-operation in this group (40.12±13.26° vs. 41.47±11.49°; p=0.174). In the TLIF group, the segmental sagittal angle slightly increased from 10.23±5.28° preoperatively to 10.44±5.28° postoperatively, whereas lumbar lordosis did not change from 41.18±10.46° preoperatively to 41.32±10.18° postoperatively (p>0.05) (Table 4).
The fusion rate was 87.7% (71 of 81 patients) at 12 months post-operation in the DLIF group, compared to 98.1% (96 of 98 patients) in the TLIF group (p=0.007).
In the DLIF group, complications related to additional transpsoas retroperitoneal approaches occurred in 16 patients (19.6%), including psoas muscle (10 patients, 12.3%), lateral femoral cutaneous nerve (4 patients, 4.9%), and genitofemoral nerve symptoms (2 patients, 2.5%). Most complications were temporary and disappeared within 2 months post-operation. In the TLIF group, infection occurred in 1 patient.
Go to : 
TLIF techniques have been used to treat various degenerative lumbar disorders over the last 3 decades. This operative method utilizes a posterior approach, which sufficiently exposes the disc space by resecting a single facet joint, reduces retraction of the thecal sac and nerve root, and preserves the contralateral structure. Furthermore, its fusion rate is high5,8,11,26).
DLIF, a different fusion method, is also widely used for degenerative lumbar disorders, and its application has been expanded. DLIF avoids the risks of thecal sac injury, arachnoiditis, and CSF fistula, and it has superior in terms of indirect decompression and sagittal and coronal restoration1,18,23). By using a retroperitoneal space approach, it is possible to insert larger interbody cages supporting bilateral epiphyseal rings, reduce tissue trauma and blood loss, decrease the operative time, and preserve the posterior longitudinal ligament complex19,23). However, the technique is associated with risks related to the transpsoas approach, including injuries to the psoas muscle, lateral femoral cutaneous nerve, geni-tofemoral nerve, and lumbosacral plexus4,10,20). In addition, patients with L5-S1 involvement are not suitable candidates for DLIF. An additional limiting factor is the requirement of additional posterior decompression when severe central spinal stenosis, uncontained disc herniation, or significant facet arthropathy is present23).
No significant differences in clinical outcomes were observed between the TLIF and DLIF groups.
The DLIF group displayed greater corrective force than the TLIF group considering the intervertebral disc and foraminal heights. This result was predictable because of the differences between the groups in terms of surgical techniques and cage features. In DLIF, there is no structure obstructing cage insertion, and the insertion of larger cages is possible because disc distraction can be performed in the disc midline. Furthermore, as the cages are located on both sides of the ring apophysis, which is the strongest part of the vertebral body, disc distraction is effective and well preserved after cage insertion. On the other hand, in TLIF, cage insertion should be performed more cautiously to avoid complications resulting from damage in adjacent areas, owing to the presence of the posterior structure and dural sac. The cage is located in concave parts of the endplate, which might be related with the ineffective increase of disc height after TLIF cage insertion.
Compared with the TLIF group, the DLIF group displayed superior outcomes considering the correction of coronal balance. However, lumbar lordosis was similar outcomes between the groups. Although there was no significant difference in the segmental sagittal angle between the groups, DLIF was more effective in causing lordosis than TLIF, as indicated by the pre- and post-operative difference in the segmental sagittal angle1). There are a growing number of studies on the correction of sagittal and coronal balances in DLIF1,14,17). Similar to our results, DLIF has become applicable in correcting various degenerative lumbar deformities in adults as well6,12,30).
The TLIF group exhibited a higher fusion rate than the DLIF group. Other studies have reported that the rate of bone fusion was 100% in patients who underwent posterolateral lumbar fusion with autologous bone, whereas in DBM, the fusion rate was 89.7%7). As a result, it appears that the fusion rate of the DLIF group who were treated with DBM was lower than that of the TLIF group with autologous bone.
In recent years, TLIF has evolved from open TLIF to minimally invasive TLIF, and DLIF has been more extensively used for deformity surgeries6,12,29,30). On the basis of our results, TLIF appears to be more appropriate for surgeries on short segments; for the treatment of well-balanced spine diseases, ruptured disc herniation, or severe central spinal stenosis; and for patients with L5-S1 involvement. DLIF appears to be more appropriate for surgeries on long segments and for cases to restore or preserve sagittal and coronal angles.
The present study has some limitations. First, the follow-up period was relatively short for evaluating long-term clinical results. Second, we did not examine the effect of the screw type or that of open or percutaneous screw systems. Third, the effect of graft materials on the fusion rate also needs to be investigated for each group. Fourth, the effect of indirect decompression was not compared between the groups. We believe prospective long-term studies are necessary for a more comprehensive evaluation in the future.
Go to : 
Both DLIF and TLIF are less invasive, good surgical options for degenerative lumbar diseases. According to our data, DLIF has higher potential in increasing neural foramina and correcting coronal balance, and involves a shorter operative time and reduced reduced EBL, in comparison with TLIF. However, DLIF displayed a lower fusion rate than TLIF, and caused additional complications related to the transpsoas approach.
Go to : 
References
1. Acosta FL, Liu J, Slimack N, Moller D, Fessler R, Koski T. Changes in coronal and sagittal plane alignment following minimally invasive direct lateral interbody fusion for the treatment of degenerative lumbar disease in adults : a radiographic study. J Neurosurg Spine. 2011; 15:92–96. PMID: 21476802.

2. Baker JK, Reardon PR, Reardon MJ, Heggeness MH. Vascular injury in anterior lumbar surgery. Spine (Phila Pa 1976). 1993; 18:2227–2230. PMID: 8278837.

3. Bridwell KH, Lenke LG, McEnery KW, Baldus C, Blanke K. Anterior fresh frozen structural allografts in the thoracic and lumbar spine. Do they work if combined with posterior fusion and instrumentation in adult patients with kyphosis or anterior column defects? Spine (Phila Pa 1976). 1995; 20:1410–1418. PMID: 7676341.

4. Cummock MD, Vanni S, Levi AD, Yu Y, Wang MY. An analysis of postoperative thigh symptoms after minimally invasive transpsoas lumbar interbody fusion. J Neurosurg Spine. 2011; 15:11–18. PMID: 21476801.

5. Cutler AR, Siddiqui S, Mohan AL, Hillard VH, Cerabona F, Das K. Comparison of polyetheretherketone cages with femoral cortical bone allograft as a single-piece interbody spacer in transforaminal lumbar interbody fusion. J Neurosurg Spine. 2006; 5:534–539. PMID: 17176018.

6. Dakwar E, Cardona RF, Smith DA, Uribe JS. Early outcomes and safety of the minimally invasive, lateral retroperitoneal transpsoas approach for adult degenerative scoliosis. Neurosurg Focus. 2010; 28:E8. PMID: 20192668.

7. Glassman SD, Carreon L, Djurasovic M, Campbell MJ, Puno RM, Johnson JR, et al. Posterolateral lumbar spine fusion with INFUSE bone graft. Spine J. 2007; 7:44–49. PMID: 17197332.

8. Hackenberg L, Halm H, Bullmann V, Vieth V, Schneider M, Liljenqvist U. Transforaminal lumbar interbody fusion : a safe technique with satisfactory three to five year results. Eur Spine J. 2005; 14:551–558. PMID: 15672243.

9. Harms J, Rolinger H. A one-stager procedure in operative treatment of spondylolistheses : dorsal traction-reposition and anterior fusion (authors transl). Z Orthop Ihre Grenzgeb. 1982; 120:343–347. PMID: 7113376.
10. Houten JK, Alexandre LC, Nasser R, Wollowick AL. Nerve injury during the transpsoas approach for lumbar fusion. J Neurosurg Spine. 2011; 15:280–284. PMID: 21619401.

11. Houten JK, Post NH, Dryer JW, Errico TJ. Clinical and radiographically/neuroimaging documented outcome in transforaminal lumbar interbody fusion. Neurosurg Focus. 2006; 20:E8. PMID: 16599424.

12. Isaacs RE, Hyde J, Goodrich JA, Rodgers WB, Phillips FM. A prospective, nonrandomized, multicenter evaluation of extreme lateral interbody fusion for the treatment of adult degenerative scoliosis : perioperative outcomes and complications. Spine (Phila Pa 1976). 2010; 35(26 Suppl):S322–S330. PMID: 21160396.
13. Jiang SD, Chen JW, Jiang LS. Which procedure is better for lumbar interbody fusion : anterior lumbar interbody fusion or transforaminal lumbar interbody fusion? Arch Orthop Trauma Surg. 2012; 132:1259–1266. PMID: 22622795.

14. Johnson RD, Valore A, Villaminar A, Comisso M, Balsano M. Pelvic parameters of sagittal balance in extreme lateral interbody fusion for degenerative lumbar disc disease. J Clin Neurosci. 2013; 20:576–581. PMID: 23375396.

15. Kawaguchi Y, Matsui H, Tsuji H. Back muscle injury after posterior lumbar spine surgery. A histologic and enzymatic analysis. Spine (Phila Pa 1976). 1996; 21:941–944. PMID: 8726197.
16. Kawaguchi Y, Yabuki S, Styf J, Olmarker K, Rydevik B, Matsui H, et al. Back muscle injury after posterior lumbar spine surgery. Topographic evaluation of intramuscular pressure and blood flow in the porcine back muscle during surgery. Spine (Phila Pa 1976). 1996; 21:2683–2688. PMID: 8961456.
17. Kepler CK, Huang RC, Sharma AK, Meredith DS, Metitiri O, Sama AA, et al. Factors influencing segmental lumbar lordosis after lateral trans psoas interbody fusion. Orthop Surg. 2012; 4:71–75. PMID: 22615150.

18. Kepler CK, Sharma AK, Huang RC, Meredith DS, Girardi FP, Cammisa FP Jr, et al. Indirect foraminal decompression after lateral transpsoas interbody fusion. J Neurosurg Spine. 2012; 16:329–333. PMID: 22284229.

19. Knight RQ, Schwaegler P, Hanscom D, Roh J. Direct lateral lumbar interbody fusion for degenerative conditions : early complication profile. J Spinal Disord Tech. 2009; 22:34–37. PMID: 19190432.
20. Le TV, Burkett CJ, Deukmedjian AR, Uribe JS. Postoperative lumbar plexus injury after lumbar retroperitoneal transpsoas minimally invasive lateral interbody fusion. Spine (Phila Pa 1976). 2013; 38:E13–E20. PMID: 23073358.

21. Lee CS, Hwang CJ, Lee DH, Kim YT, Lee HS. Fusion rates of instrumented lumbar spinal arthrodesis according to surgical approach : a systematic review of randomized trials. Clin Orthop Surg. 2011; 3:39–47. PMID: 21369477.

22. McAfee PC, Regan JJ, Geis WP, Fedder IL. Minimally invasive anterior retroperitoneal approach to the lumbar spine. Emphasis on the lateral BAK. Spine (Phila Pa 1976). 1998; 23:1476–1484. PMID: 9670400.

23. Oliveira L, Marchi L, Coutinho E, Pimenta L. A radiographic assessment of the ability of the extreme lateral interbody fusion procedure to indirectly decompress the neural elements. Spine (Phila Pa 1976). 2010; 35:S331–S337. PMID: 21160397.

24. Ozgur BM, Aryan HE, Pimenta L, Taylor WR. Extreme Lateral Interbody Fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J. 2006; 6:435–443. PMID: 16825052.

25. Park JS, Kim YB, Hong HJ, Hwang SN. Comparison between posterior and transforaminal approaches for lumbar interbody fusion. J Korean Neurosurg Soc. 2005; 37:340–344.
26. Potter BK, Freedman BA, Verwiebe EG, Hall JM, Polly DW Jr, Kuklo TR. Transforaminal lumbar interbody fusion : clinical and radiographic results and complications in 100 consecutive patients. J Spinal Disord Tech. 2005; 18:337–346. PMID: 16021015.
27. Rajaraman V, Vingan R, Roth P, Heary RF, Conklin L, Jacobs GB. Visceral and vascular complications resulting from anterior lumbar interbody fusion. J Neurosurg. 1999; 91(1 Suppl):60–64. PMID: 10419370.

28. Sasso RC, Kenneth Burkus J, LeHuec JC. Retrograde ejaculation after anterior lumbar interbody fusion transperitoneal versus retroperitoneal exposure. Spine (Phila Pa 1976). 2003; 28:1023–1026. PMID: 12768143.
29. Tian NF, Wu YS, Zhang XL, Xu HZ, Chi YL, Mao FM. Minimally invasive versus open transforaminal lumbar interbody fusion : a meta-analysis based on the current evidence. Eur Spine J. 2013; 22:1741–1749. PMID: 23572345.

30. Tormenti MJ, Maserati MB, Bonfield CM, Okonkwo DO, Kanter AS. Complications and radiographic correction in adult scoliosis following combined transpsoas extreme lateral interbody fusion and posterior pedicle screw instrumentation. Neurosurg Focus. 2010; 28:E7. PMID: 20192667.

31. Zhou ZJ, Zhao FD, Fang XQ, Zhao X, Fan SW. Meta-analysis of instrumented posterior interbody fusion versus instrumented posterolateral fusion in the lumbar spine. J Neurosurg Spine. 2011; 15:295–310. PMID: 21619404.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download