Abstract
Cerebral hyperperfusion syndrome (CHS) is increasingly recognized as an uncommon, but serious, complication subsequent to carotid artery stenting (CAS) and carotid endarterectomy (CEA). The onset of CHS generally occurs within two weeks of CEA and CAS, and a delay in the onset of CHS of over one week after CAS is quite rare. We describe a patient who developed CHS three weeks after CAS with status epilepticus.
Cerebral hyperperfusion syndrome (CHS) is a dangerous complications following carotid endarterectomy (CEA) or carotid artery stenting (CAS)3,4,7,11). CHS following CEA or CAS is characterized by unilateral headache, seizure, focal neurologic defects, and intracerebral hemorrhage in most severe form6,9). Little is known about the exact cause of CHS; however, a deterioration in cerebrovascular autoregulation seems to increase regional cerebral blood and lead to CHS1).
Rate of occurrence of CHS after CAS is reported to be 0.4% to 3%1,8,9). Previous studies found that CHS mostly occurs within one week after CAS6,9). Although there are a few reports of CHS occurring from three weeks to four weeks after CEA, a delay in onset of over one week is very rare6). Risk factors for CHS are known diabetes mellitus, old age, recent contralateral CEA, post-interventional hypertension, contralateral carotid occlusion, intra-interventional ischemia, and administration of anticoagulants or antiplatelet agents.
We describe a case of delayed CHS, presenting as status epilepticus, three weeks after CAS in a high risk patient.
A 67-year-old woman was admitted for evaluation of an incidental finding of carotid bruit on physical examination. She had been treated for hypertension and diabetes mellitus for 10 years. On admission, her blood pressure was 140/100 mg. On neurological examination, she was normal. Magnetic resonance imaging (MRI) revealed multiple old infarctions in the bilateral parieto-occipital junctions and right internal border zone area. MR angiography revealed occlusion of the right proximal internal carotid artery (ICA), and severe stenosis of the left proximal ICA and orifices of both vertebral arteries (VA). Digital subtraction angiography demonstrated total occlusion of the right proximal ICA, 73% stenosis of the left proximal ICA and more than 70% stenosis of both VA orifices (Fig. 1A). The patient was pre-treated with aspirin and clopidogrel for 5 days before undergoing stenting at the left proximal ICA and right VA orifices via a transfemoral approach under local anesthesia (Fig. 1B). There was no transient hypotension or bradycardia during the periprocedural period. Anti-hypertensive drugs including a calcium channel blocker (CCB) were administered to maintain systolic blood pressure below 140 mm Hg. After close observation for one week, she was discharged without any complications.
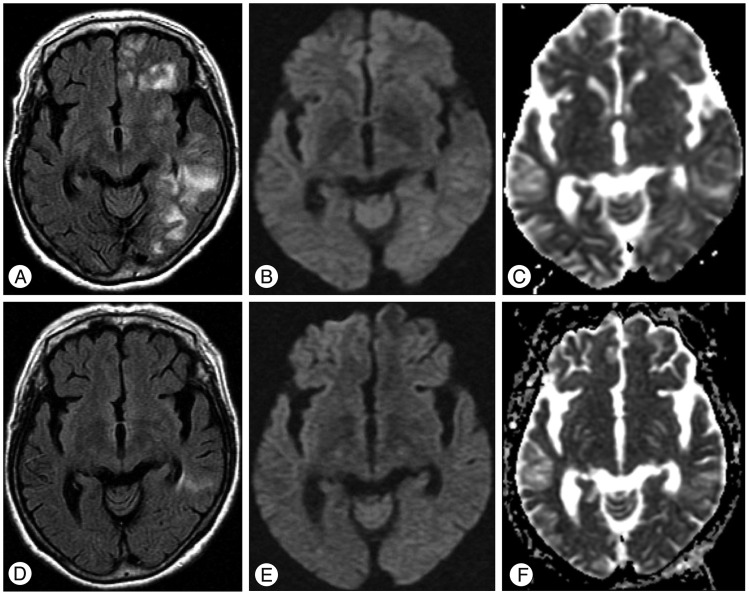
Three weeks after CAS, the patient presented to the emergency room with generalized tonic-clonic seizure for 40 minutes and stuporous mentality. Blood pressure had risen to 190/110 mm Hg. On neurologic examination, the withdrawal response in the right extremities was decreased, but there was hyper-reflexia in all the extremities, with pathologic plantar reflexes bilaterally. Brain MRI showed high signal intensities in the left hemispheric white matter on FLAIR, without diffusion restriction, pointing to vasogenic edema (Fig. 2A, B, C). Neck computed tomography angiography revealed no significant stenosis in the previously stented vessel. Slower waves were recorded on the left than on the right hemisphere on electroencephalograms, and there was no epileptiform discharge. Thees findings were compatible with CHS presenting vasogenic edematous change with symptomatic epilepsy. After administration of antiepileptics and antihypertensives, the deteriorated mentality recovered slowly and seizures ceased. The weakness of the right extremities recovered gradually from the sixth hospital day.
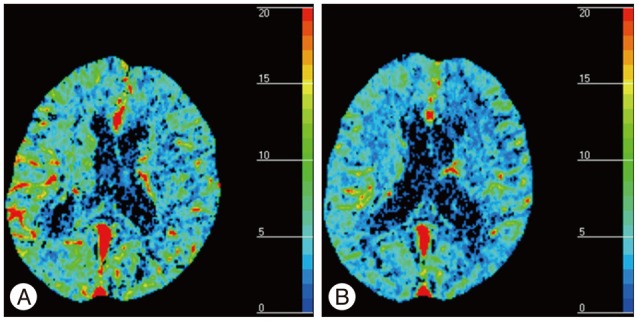
The patient's blood pressure was controlled by high dose intravenous labetalol and losartan/hydrochlorothiazide. Follow-up MRI of two weeks after CHS showed resolution of the vasogenic edema (Fig. 2D, E, F). Perfusion CT was performed at the time of symptom development and 24 days after CHS, when the state of the CHS had improved. It revealed a higher cerebral blood volume (CBV) in both hemispheres at the onset than 24 days after CHS. The CBV returned to normal within 4 weeks (Fig. 3) and the patient recovered without any neurological abnormality. Blood pressure was normalized by the prescribed drugs and the seizure was stabilized with valproic acid and levetiracetam.
There have been a few reports of CHS after CEA, but it has rarely been described after CAS6). The pathophysiology of CHS after CAS remains unclear, but there are several possible mechanisms. Firstly, prolonged hypoperfusion may lead to abnormality of cerebral vascular autoregulation8). Secondly, transient bradycardia and hypotension due to damage to the carotid artery baroreceptor can often occur during CAS, and can result in further ischemic injury to damaged brains9). Thirdly, systemic hypertension secondary to CAS can result in intense cerebral blood flow1,6).
The most important issue in this case is differentiation between CHS and seizure due to a periprocedural thromboembolism. We performed perfusion CT at the time of symptom development and 24 days after CHS when the CHS had improved. In the perfusion CT, greater CBV was observed in both hemispheres at the onset of the CHS than 24 days after the CHS. Periprocedural thromboembolism is a common adverse effect of CAS and a risk factor for CHS. If the patient had presented with seizure due to a periprocedural embolism, the features of the perfusion CT scan might have been different. The most common post-ictal perfusion abnormality is decreased cerebral blood flow and cerebral blood volume2). CT perfusion revealed a reduced time-to-peak and mean-transient-time and increased cerebral blood volume and cerebral blood flow in the CHS10). The perfusion CT scan was compatible with CHS, and the patient had no neurological deficit or clinical symptoms related to the CHS, based on the results of blood pressure measurements over the 2 weeks after the CHS. Although we did not perform a special evaluation, the possibility of a diagnosis of periprocedural thromboembolism or other adverse effect seemed to be low. Also, the large lesion and generalized clinical symptoms were more likely to be related to CHS than to a thromboembolism.
In the present case, the occurrence of CHS was associated with multiple risk factors including ipsilateral occlusion, contralateral stenosis, poor collateral circulation, old age, long-standing hypertension, and administration of antiplatelet agents9,13). Although the patient had potential risk factors for CHS, the latter could have been due to some other mechanism. One might the usage of CCB, which could induce cerebral vasodilation5,12). Another might be associated with the unstable status of preprocedural cerebrovascular reactivity in patients with various predisposing factors11), or the unknown status of postprocedural cerebrovascular autoregulation11).
This case suggests that we should take into careful consideration all periprocedural risk factors for CHS. In addition, it suggests that delayed onset of CHS may occasionally present with status epilepticus. After CAS, clinicians should observe patients who have undergone CAS and have high risk factors for at least two or three weeks.
Acknowledgements
This study was supported by the cluster research fund of Hanyang University (HY-2009-C).
References
1. Abou-Chebl A, Yadav JS, Reginelli JP, Bajzer C, Bhatt D, Krieger DW. Intracranial hemorrhage and hyperperfusion syndrome following carotid artery stenting : risk factors, prevention, and treatment. J Am Coll Cardiol. 2004; 43:1596–1601. PMID: 15120817.

2. Gelfand JM, Wintermark M, Josephson SA. Cerebral perfusion-CT patterns following seizure. Eur J Neurol. 2010; 17:594–601. PMID: 19968701.

3. Ho DS, Wang Y, Chui M, Ho SL, Cheung RT. Epileptic seizures attributed to cerebral hyperperfusion after percutaneous transluminal angioplasty and stenting of the internal carotid artery. Cerebrovasc Dis. 2000; 10:374–379. PMID: 10971023.

4. Kim DE, Choi SM, Yoon W, Kim BC. Hyperperfusion syndrome after carotid stent-supported angioplasty in patients with autonomic dysfunction. J Korean Neurosurg Soc. 2012; 52:476–479. PMID: 23323169.

5. Kuriyama Y, Hashimoto H, Nagatsuka K, Sawada T, Omae T. Effects of dihydropyridines on cerebral blood vessels. J Hypertens Suppl. 1993; 11:S9–S12. PMID: 8169383.

6. Lieb M, Shah U, Hines GL. Cerebral hyperperfusion syndrome after carotid intervention : a review. Cardiol Rev. 2012; 20:84–89. PMID: 22183061.
7. McCabe DJ, Brown MM, Clifton A. Fatal cerebral reperfusion hemorrhage after carotid stenting. Stroke. 1999; 30:2483–2486. PMID: 10548688.

8. Moulakakis KG, Mylonas SN, Sfyroeras GS, Andrikopoulos V. Hyperperfusion syndrome after carotid revascularization. J Vasc Surg. 2009; 49:1060–1068. PMID: 19249185.

9. Ogasawara K, Sakai N, Kuroiwa T, Hosoda K, Iihara K, Toyoda K, et al. Intracranial hemorrhage associated with cerebral hyperperfusion syndrome following carotid endarterectomy and carotid artery stenting : retrospective review of 4494 patients. J Neurosurg. 2007; 107:1130–1136. PMID: 18077950.

10. Schoknecht K, Gabi S, Ifergane G, Friedman A, Shelef I. Detection of cerebral hyperperfusion syndrome after carotid endarterectomy with CT Perfusion. J Neuroimaging. 2012; [Epub ahead of print].

11. Sundt TM Jr, Sharbrough FW, Piepgras DG, Kearns TP, Messick JM Jr, O'Fallon WM. Correlation of cerebral blood flow and electroencephalographic changes during carotid endarterectomy : with results of surgery and hemodynamics of cerebral ischemia. Mayo Clin Proc. 1981; 56:533–543. PMID: 7266064.
12. Tietjen CS, Hurn PD, Ulatowski JA, Kirsch JR. Treatment modalities for hypertensive patients with intracranial pathology : options and risks. Crit Care Med. 1996; 24:311–322. PMID: 8605807.

13. van Mook WN, Rennenberg RJ, Schurink GW, van Oostenbrugge RJ, Mess WH, Hofman PA, et al. Cerebral hyperperfusion syndrome. Lancet Neurol. 2005; 4:877–888. PMID: 16297845.

Fig. 1
Pre-stenting cerebral angiography shows the narrow left proximal internal carotid artery (ICA) (A) and post-stenting cerebral angiography shows the widened left ICA stenosis (B).
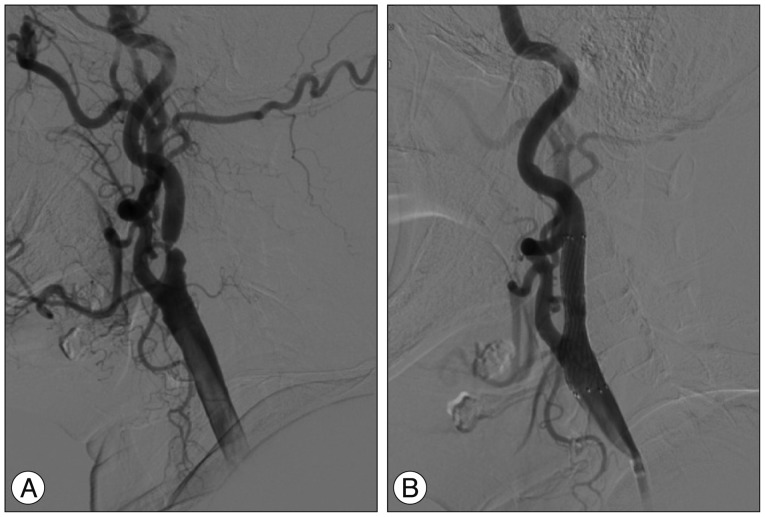
Fig. 2
Postprocedural magnetic resonance imaging three weeks (A, B, and C) and five weeks (D, E, and F) after carotid artery stenting. The signal abnormality on the fluid-attenuated inversion recovery image at three weeks (A) was markedly improved at five weeks (D). The diffusion weighted images and apparent diffusion coefficient map images shown were improved compared to the previous MR images.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download