Abstract
Reversible cerebral vasoconstriction syndrome (RCVS) is characterized by sudden-onset headache with focal neurologic deficit and prolonged but reversible multifocal narrowing of the distal cerebral arteries. Stroke, either hemorrhagic or ischemic, is a relatively frequent presentation in RCVS, but progressive manifestations of subarachnoid hemorrhage, intracerebral hemorrhage, cerebral infarction in a patient is seldom described. We report a rare case of a 56-year-old woman with reversible cerebral vasoconstriction syndrome consecutively presenting as cortical subarachnoid hemorrhage, intracerebral hemorrhage, and cerebral infarction. When she complained of severe headache with subtle cortical subarachnoid hemorrhage, her angiography was non-specific. But, computed tomographic angiography showed typical angiographic features of this syndrome after four days. Day 12, she suffered mental deterioration and hemiplegia due to contralateral intracerebral hematoma, and she was surgically treated. For recurrent attacks of headache, medical management with calcium channel blockers has been instituted. Normalized angiographic features were documented after 8 weeks. Reversible cerebral vasoconstriction syndrome should be considered as differential diagnosis of non-aneurysmal subarachnoid hemorrhage, and repeated angiography is recommended for the diagnosis of this under-recognized syndrome.
In 1988, Call et al.5) described clinical and angiographic findings in four patients with thunderclap headache related to reversible vasoconstriction of cerebral arteries. Since then, some authors have reported that reversible cerebral vasoconstriction syndrome (RCVS) is characterized by severe headaches, often thunderclap nature, with or without acute neurological symptoms, associated with multifocal segmental vasoconstriction of cerebral arteries, which resolve spontaneously within 3 months3). There have been increasing numbers of reports of RCVS, attributed to routine check-up of catheter angiography, magnetic resonance (MR) imaging or computed tomography (CT) angiography for awareness of angiographic variations. The RCVS has been often manifested hemorrhagic or ischemic stroke including cortical subarachnoid hemorrhage (SAH), intracerebral hemorrhage (ICH), and cerebral infarction3,6,7,17). However, the gradual emergence or coexistence of cortical SAH, ICH, and cerebral infarction in RCVS is seldom described. Moreover, delayed angiographic diagnosis in RCVS has been reported only a few cases1,14). Here, we report a case of SAH and ICH, and cerebral infarction-associated with delayed diagnosed RCVS which treated with surgical and medical management.
A 56-year-old female presented with an acute onset of severe headache after coughing described as "a disaster for the first time in my life", associated with mild nausea. There was no focal neurologic deficit. The patient's blood pressure was 150/90 mm Hg. There was no previous history of migraine or other type of headaches, and she denied any regular medication. Initially, head CT scan revealed isolated cortical SAH within the sulcus of right high frontal lobe (Fig. 1A). No cerebral vasoconstriction, venous sinus thrombosis, cerebral aneurysms or arteriovenous malformations were found on digital subtraction angiography (DSA) and CT angiography performed immediately after admission (Fig. 1B). After four days, CT angiography revealed multiple segments of irregularity consisting of narrowed areas of both middle cerebral arteries (M2 tracts) and right posterior cerebral artery (P2 tract) with no evidence of arterial vasoconstriction at proximal cisternal segment indicating SAH-related vasospasm (Fig. 2). However, no aneurysms or arteriovenous malformation were identified.
Physical and neurological examination findings were normal, and ophthalmologic examination excluded the possibility of retinal vasculitis or inflammation. Blood chemistry workup including coagulation function test, hormonal workup, hepatic and renal function test, and serum immunological screening for rheumatologic disease or vasculitis were all negative or within normal limits. Cerebrospinal fluid analysis was near normal except for the presence of red blood cells indicating SAH. There was neither leukocytosis nor increased protein levels typical of primary angiitis of the central nervous system (PACNS). Transthoracic echocardiography and electrocardiography showed no definitive wall motion abnormality or arrhythmia. Intravenous nimodipine for vasodilatation with blood pressure control was applied under suspicion of RCVS, and oral analgesics were prescribed for her severe headache. Her symptoms improved over the next three days.
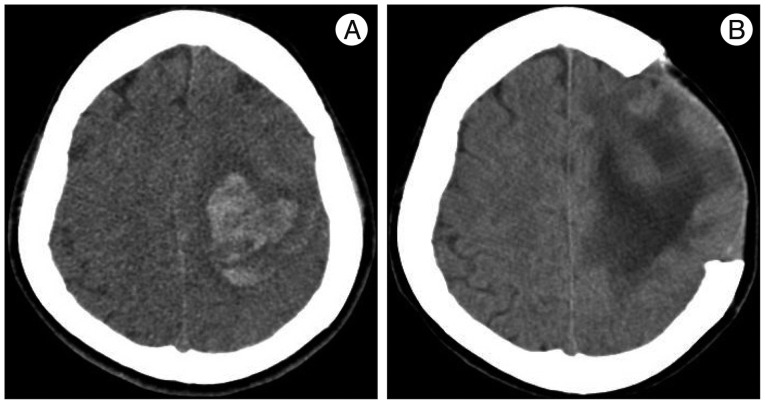
Five days later, she suffered deterioration of neurological status, stuporous mental change and motor weakness of the right side extremities after coughing. Her blood pressure was checked up to 200/120 mm Hg. CT scan showed huge intracerebral hematoma on left cerebral hemisphere, opposite to the first SAH presentation (Fig. 3A). There was no newly developed lesion in CT angiography. Emergent decompressive craniectomy and hematoma removal was performed, and she was transferred to the intensive care unit for close observation. Intravenous nimodipine was used for blood pressure control and treatment of RCVS, combined with mannitolization for intracranial pressure control.
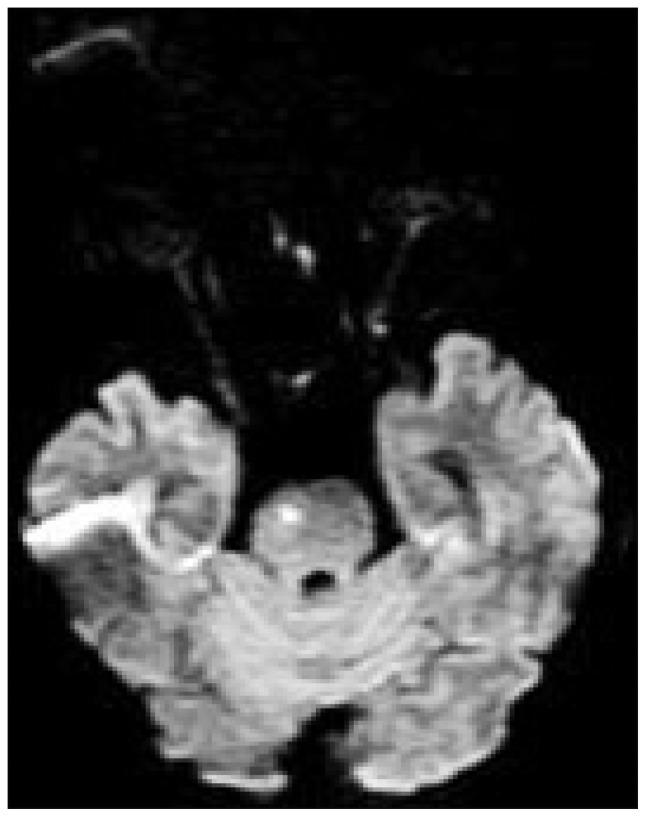
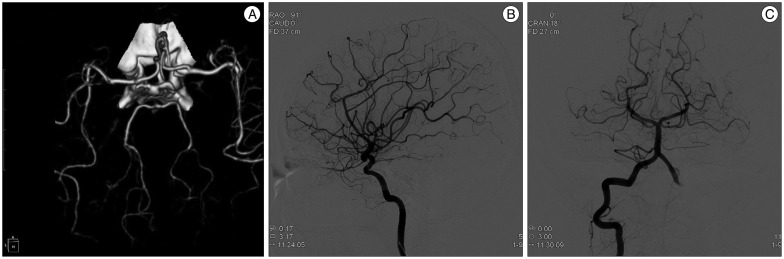
After 12 day, CT scan showed reduction of cerebral edema and hematoma (Fig. 3B). Her mental status gradually improved to drowsy, and she was transferred to general ward and then received oral amlodipine mesylate. But, her hemiplegia did not change and motor aphasia was found according to improvement of mental status. She complained intermittent mild headache, improved with analgesics. On day 30, she was transferred to department of rehabilitation for efficient recovery of motor and speech function. One week thereafter, she experienced sudden-onset headache of moderate degree and nausea not severe than her first symptoms, and brain MRI showed hyperintense signals in the pons on diffusion-weighted imaging, which are suggestive of acute to subacute cerebral infarction (Fig. 4). Without additional treatment, this event was settled uneventfully. After 6 weeks and 8 weeks on admission, CT angiography and DSA exhibited marked resolution of vasoconstriction (Fig. 5). By the end of three months, there was no evidence of recurrent symptoms and signs.
RCVS is presented radiologically and pathophysiologically by diffuse segmental constriction of cerebral arteries that spontaneously resolves within 3 months3). Although RCVS considered idiopathic, more than half of cases occur after exposure to sympathomimetic or serotonergic drugs, postpartum state, and headache disorders3,17). Sudden-onset headache is the most frequent clinical presentation and nausea, vomiting, photophobia, seizures, focal neurologic deficits can also occur.
Persistent neurologic deficits, including hemiplegia, hemianopia, aphasia from stroke or severe sustained cerebral vasoconstriction have also been described9,17). Some authors reported that the most important factors influencing outcome are related to complications of intraparenchymal hemorrhage11,13,19). Pathophysiologically, there are multiple factors causing hemorrhages including pial vessel rupture or reperfusion injuries due to high pressure in the narrowed vessels (caused by a sudden increase in blood pressure) combined with impairment of cerebral autoregulation10,16). However, ischemic complication is developed by involvement of more proximal medium- or large-sized arteries after distal small-sized arteries are affected. Segmental constriction of proximal vessels (vertebrobasilar system) might have been sustained, and this was progressed to the pontine infarction in the present case, albeit infrequently.
Spontaneous acute cortical SAH observed at the convexity of the brain is a relatively rare entity, seldom described15,18,20). An RCVS accompanying cortical SAH is relatively rare, so it can be easily underestimated or missed because of subtle presentation on CT scan. In the current case, clinical, laboratory, and radiologically findings showed no cerebrovascular abnormality in initial examination, so we could not exclude non-vasculitic cause of isolated cortical SAH. Typically, segmental vasoconstriction in RCVS occurs earlier, but, in this case, angiographic abnormalities developed later, when SAH had already occurred. Although this delayed appearance of typical angiographic abnormalities was quite rare in RCVS, there is an increasingly description of cases with a delay between clinical onset and angiographic changes1,7,13,14). Therefore, meticulous interpretation of radiologic data based on repeated DSA or CT angiography is mandated for the accurate diagnosis. Recognition of RCVS as a cause of spontaneous, non-traumatic SAH may be confused for aneurysmal SAH related vasospasm or primary angiitis of central nervous system (PACNS). In RCVS, associated vasospasm may be distinguished from SAH related vasospasm : absence of ruptured aneurysm; disproportionate distribution related to focal blood clot; segmental vasoconstriction occurring earlier (<4 days); prolonged, but reversible aspect of angiographic vasoconstriction3,4,9,10). Although the proximal cisternal segments are mainly affected in SAH related vasospasm, vascular involvement of RCVS started at small distal arteries progressing to medium- and large-sized arteries. This point may be related to progressive, gradual manifestation of variable disease entity in the present case.
Differential diagnosis of RCVS including PACNS is vague and non-specific, and not straightforward but, affects treatment option which in turn, seriously relates to clinical outcome or complications. Moreover, there are many undesirable side effects, for example, adverse effect of drugs (cytotoxic agents or high dose corticosteroids), unnecessary brain parenchymal biopsy, and repetition of expensive laboratory tests1). Angiographic reversibility within 12 weeks distinguishes RCVS from PACNS. In addition, pertinent clinical assessment is important for differential diagnosis, which has been reported as acute, self-limited course in RCVS than PACNS3,12). Cerebrospinal fluid analysis of patients with RCVS is commonly within normal limit. However, the result from cerebrospinal fluid study in patients with PACNS typically demonstrates pleocytosis with elevated protein levels and leukocytosis4,12).
There is no established treatment protocol of RCVS. Authors reported, calcium channel blockers or short term glucocorticoids medications are reported to be effective for treatment of RCVS4,7,9,11). We used nimodipine via intravenous route after angiographic diagnosis. Although headache improved over several days, huge intracerebral hemorrhage occurred in the interim period that requiring surgery. We continued the prescription for the treatment of vasoconstriction and arterial hypertension. The patient experienced recurrent symptom with pontine infarction, but, tolerated well and gradually recovered. However, if we were familiar with predictable factor to progress a severe presentations or there was prompt diagnosis, intra-arterial administration of calcium channel blockers as vasodilator via endovascular technique might have been effective2,8) or played a preventive role against hemorrhagic transformations. Hemorrhagic transformations of RCVS can be developed by rupture of arterioles due to vasoconstriction-induced high pressure in narrowed vessels.
The combination of cortical SAH, ICH, and cerebral infarction with the course of the time in the present case which were recognized with delayed, angiographic evidence of RCVS, teaches important messages. Stroke, either hemorrhagic or ischemic, is related to clinical outcome. RCVS should be considered as differential diagnosis of non-aneurysmal SAH, especially in isolated cortical SAH and repeated angiography is recommended for the diagnosis of this under-recognized syndrome, RCVS.
References
1. Ansari SA, Rath TJ, Gandhi D. Reversible cerebral vasoconstriction syndromes presenting with subarachnoid hemorrhage : a case series. J Neurointerv Surg. 2011; 3:272–278. PMID: 21990840.

2. Bouchard M, Verreault S, Gariépy JL, Dupré N. Intra-arterial milrinone for reversible cerebral vasoconstriction syndrome. Headache. 2009; 49:142–145. PMID: 18647181.

3. Calabrese LH, Dodick DW, Schwedt TJ, Singhal AB. Narrative review : reversible cerebral vasoconstriction syndromes. Ann Intern Med. 2007; 146:34–44. PMID: 17200220.

4. Calabrese LH, Gragg LA, Furlan AJ. Benign angiopathy : a distinct subset of angiographically defined primary angiitis of the central nervous system. J Rheumatol. 1993; 20:2046–2050. PMID: 8014931.
5. Call GK, Fleming MC, Sealfon S, Levine H, Kistler JP, Fisher CM. Reversible cerebral segmental vasoconstriction. Stroke. 1988; 19:1159–1170. PMID: 3046073.

6. Chen SP, Fuh JL, Lirng JF, Chang FC, Wang SJ. Recurrent primary thunderclap headache and benign CNS angiopathy : spectra of the same disorder. Neurology. 2006; 67:2164–2169. PMID: 17190937.

7. Ducros A, Fiedler U, Porcher R, Boukobza M, Stapf C, Bousser MG. Hemorrhagic manifestations of reversible cerebral vasoconstriction syndrome : frequency, features, and risk factors. Stroke. 2010; 41:2505–2511. PMID: 20884871.

8. Elstner M, Linn J, Müller-Schunk S, Straube A. Reversible cerebral vasoconstriction syndrome : a complicated clinical course treated with intra-arterial application of nimodipine. Cephalalgia. 2009; 29:677–682. PMID: 19239677.

9. Hajj-Ali RA, Furlan A, Abou-Chebel A, Calabrese LH. Benign angiopathy of the central nervous system : cohort of 16 patients with clinical course and long-term followup. Arthritis Rheum. 2002; 47:662–669. PMID: 12522842.

10. Hantson P, Forget P. Reversible cerebral vasospasm, multilobular intracerebral hemorrhages, and nonaneurysmal subarachnoid hemorrhage : review of possible interrelationships. Curr Pain Headache Rep. 2010; 14:228–232. PMID: 20425193.

11. Moskowitz SI, Calabrese LH, Weil RJ. Benign angiopathy of the central nervous system presenting with intracerebral hemorrhage. Surg Neurol. 2007; 67:522–527. discussion 527-528. PMID: 17445624.

12. Nadeau SE. Diagnostic approach to central and peripheral nervous system vasculitis. Neurol Clin. 1997; 15:759–777. PMID: 9367963.

13. Noskin O, Jafarimojarrad E, Libman RB, Nelson JL. Diffuse cerebral vasoconstriction (Call-Fleming syndrome) and stroke associated with antidepressants. Neurology. 2006; 67:159–160. PMID: 16832100.

14. Quartuccio L, Tuniz F, Petralia B, Zanotti B, Skrap M, Vita SD. Delayed positivization of cerebral angiography in reversible cerebral vasoconstriction syndrome (RCVS) presenting with recurrent subarachnoid haemorrhage. Open Rheumatol J. 2012; 6:175–179. PMID: 22870164.

15. Refai D, Botros JA, Strom RG, Derdeyn CP, Sharma A, Zipfel GJ. Spontaneous isolated convexity subarachnoid hemorrhage : presentation, radiological findings, differential diagnosis, and clinical course. J Neurosurg. 2008; 109:1034–1041. PMID: 19035716.

16. Shah AK. Non-aneurysmal primary subarachnoid hemorrhage in pregnancy-induced hypertension and eclampsia. Neurology. 2003; 61:117–120. PMID: 12847171.

17. Singhal AB, Hajj-Ali RA, Topcuoglu MA, Fok J, Bena J, Yang D, et al. Reversible cerebral vasoconstriction syndromes : analysis of 139 cases. Arch Neurol. 2011; 68:1005–1012. PMID: 21482916.
18. Spitzer C, Mull M, Rohde V, Kosinski CM. Non-traumatic cortical subarachnoid haemorrhage : diagnostic work-up and aetiological background. Neuroradiology. 2005; 47:525–531. PMID: 15971064.

19. Sturm JW, Macdonell RA. Recurrent thunderclap headache associated with reversible intracerebral vasospasm causing stroke. Cephalalgia. 2000; 20:132–135. PMID: 10961771.

20. van Gijn J, Rinkel GJ. Subarachnoid haemorrhage : diagnosis, causes and management. Brain. 2001; 124(Pt 2):249–278. PMID: 11157554.
Fig. 1
A : Initial head CT scan shows subtle cortical subarachnoid hemorrhage overlying the right high frontal convexity. B : Digital subtraction angiography shows no aneurysms or arteriovenous malformation or other arterial caliber change.
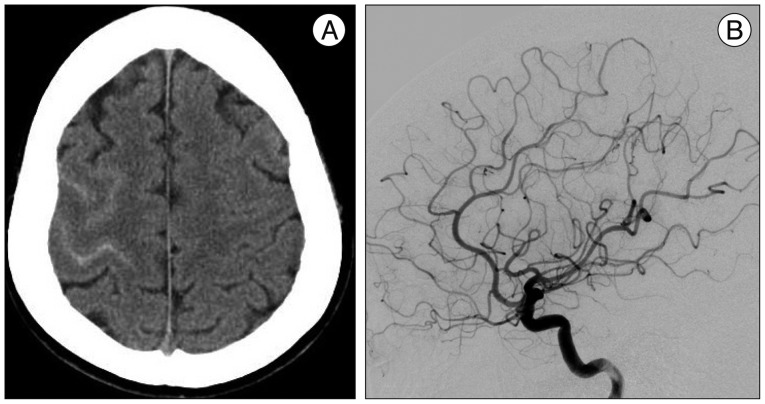
Fig. 2
Repeated CT angiographic images after 4 days on admission demonstrate prominent multifocal segmental vasoconstrictions involving superior division of both middle cerebral arteries (M2 tracts) and right posterior cerebral artery (P2 tract).
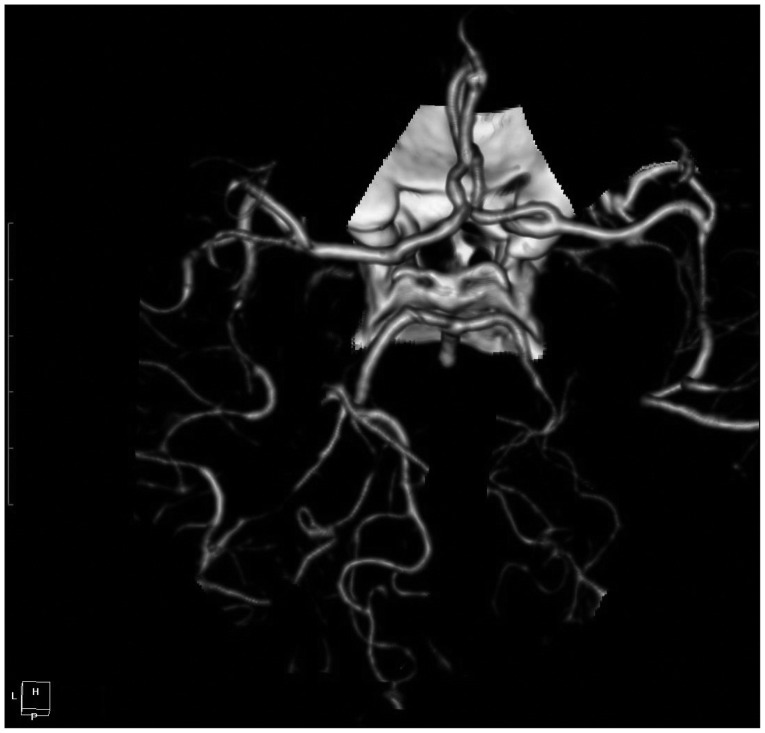
Fig. 3
A : Non-enhanced head CT at day 12 after admission shows intracerebral hemorrhage with edema on left cerebral hemisphere, associated with neurologic deterioration of the patient. B : On postoperative 12th day, CT scan reveals decreased extent of edema and hemorrhage.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download