Abstract
Objective
To determine the efficacy of endoscopic surgery in combination with long-acting somatostatin analogues (SSAs) in treating patients with growth hormone (GH)-secreting pituitary tumor.
Methods
We performed retrospective analysis of 133 patients with GH producing pituitary adenoma who underwent pure endoscopic transsphenoidal surgery in our center from January 2007 to July 2012. Patients were followed up for a range of 3-48 months. The radiological remission, biochemical remission and complication were evaluated.
Results
A total of 110 (82.7%) patients achieved radiological complete resection, 11 (8.2%) subtotal resection, and 12 (9.0%) partial resection. Eighty-eight (66.2%) patients showed nadir GH level less than 1 ng/mL after oral glucose administration. No mortality or severe disability was observed during follow up. Preoperative long-acting SSA successfully improved left ventricle ejection fraction (LVEF) and blood glucose in three patients who subsequently underwent success operation. Long-acting SSA (20 mg every 30 days) achieved biochemical remission in 19 out 23 (82.6%) patients who showed persistent high GH level after surgery.
Untreated acromegaly has been associated with reduced life expectancy because of high level of growth hormone (GH) and insulin growth hormone 1 (IGF-1)14). Timely and optimized treatment, therefore, is critical to improve patient outcome. Therapeutic options include surgery, medical therapy and radiotherapy. Transsphenoidal microsurgery, which can rapidly control GH/IGF-1 level, remains the first line treatment for GH-secreting adenomas2,8,10). Somatostatin analogs represent useful options mainly for tumors with small chance of surgical success6). However, the costs and frequent injection due to short half-life limit their application.
Recent years have witnessed advances in the treatment of GH producing pituitary adenoma. The use of endoscope, intraoperative magnetic resonance imaging (iMRI) and neuro-navigation system has increased cure rate and decreased complication rate. Postoperative suppressive therapy by somatostatin analogue achieved significantly higher rate of biochemical remission6). The Acromegaly Consensus Group (ACG) has published several consensuses on acromegaly management since 2000, in regarding as diagnosis, treatment and remission criteria. In 2011, the American Association of Clinical Endocrinologists (AACE) updated the guidelines for clinical practice for diagnosis and treatment of acromegaly8). Though studies have found endoscopic approach is associated with a high rate of remission based on the most updated criteria and guidelines1,11), data from large population from China is lacking.
Therefore, we retrospectively analyzed a total of 133 patients with GH-secreting pituitary tumor treated by endoscopic transsphenoidal surgery and SSA by a single surgeon in our institute from 2007 to 2012.
A constitutive of 133 patients with GH producing pituitary adenoma who underwent endoscopic transsphenoidal surgery by a single surgeon at Department of Neurosurgery, our hospital between January 2007 and July 2012 were enrolled in this study. Patients were diagnosed as GH producing pituitary adenoma when fulfilled all the following criteria : clinical manifestations of acromegaly or gigantism; random GH >2.5 µg/L, OGTT nadir GH >1.0 µg/L, IGF-1 >age-corrected reference value; sellar region tumor detected by CT and MRI; pituitary growth hormone adenoma confirmed by post-surgery pathology. Those with negative manifestations of acromegaly or gigantism but positive histological finding of GH staining were not included in this study. This study was approved by our institutional review board and all patients signed consented form.
Since 2007 pure endoscopic transsphenoidal surgery has been applied to replace microscope transsphenoidal surgery in the treatment of GH producing pituitary adenoma in our center. In the current study, all patients underwent a purely endoscopic transsphenoidal approach, which included a single or binarial three-hand technique with wide anterior sphenoidotomy and partial posterior septectomy without middle turbinectomy. When tumor invasion was detected in sphenoidal sinus and clivus, the surrounding dura should be removed. Instruments included 4 mm-diameter rigid neuroendoscopy with automatic flushing pump (Storz, Germany); Sony monitor and video image acquisition system; 1.5 T iMRI (Calgary Crane System, IMRIS); and Medtronic or Brain Lab neruo-navigation system. 1.5 T iMRI was used 15 cases of macro adenoma (>3 cm) or giant adenoma (>4 cm); neuro-navigation system was adopted in 51 cases.
All patients were required to take contrast-enhanced MR imaging at three months after surgery and at a yearly interval afterward. Serum level of GH and IGF-1 was measured preoperatively and at each visit postoperatively. The patients were 100% fol-lowed at the 3rd month after surgery but subsequently 19 patients were lost to follow-up. Patients were followed up for a range of 3 to 48 month.
Preoperative SSA therapy was given to three patients with heart failure and unsuitable for operation. Surgery was performed when cardiac function was improved after 1-3 months of SSA treatment. For those who failed to achieve GH control after surgery, long-acting SSA (20 mg every 30 days) was administered irrespective of residual tumor detected by MRI.
The purpose of treatment was to completely remove the tumor without causing hypopituitarism. The tumor was considered to be totally removed when MRI reported negative tumor at the 3rd month after surgery. The biochemical "cure" was defined as following : the nadir GH level after oral glucose administration should be less than 1 ng/mL, and the IGF-I level should correspond to the appropriate age- and sex-adjusted reference values according to AACE guidelines8).
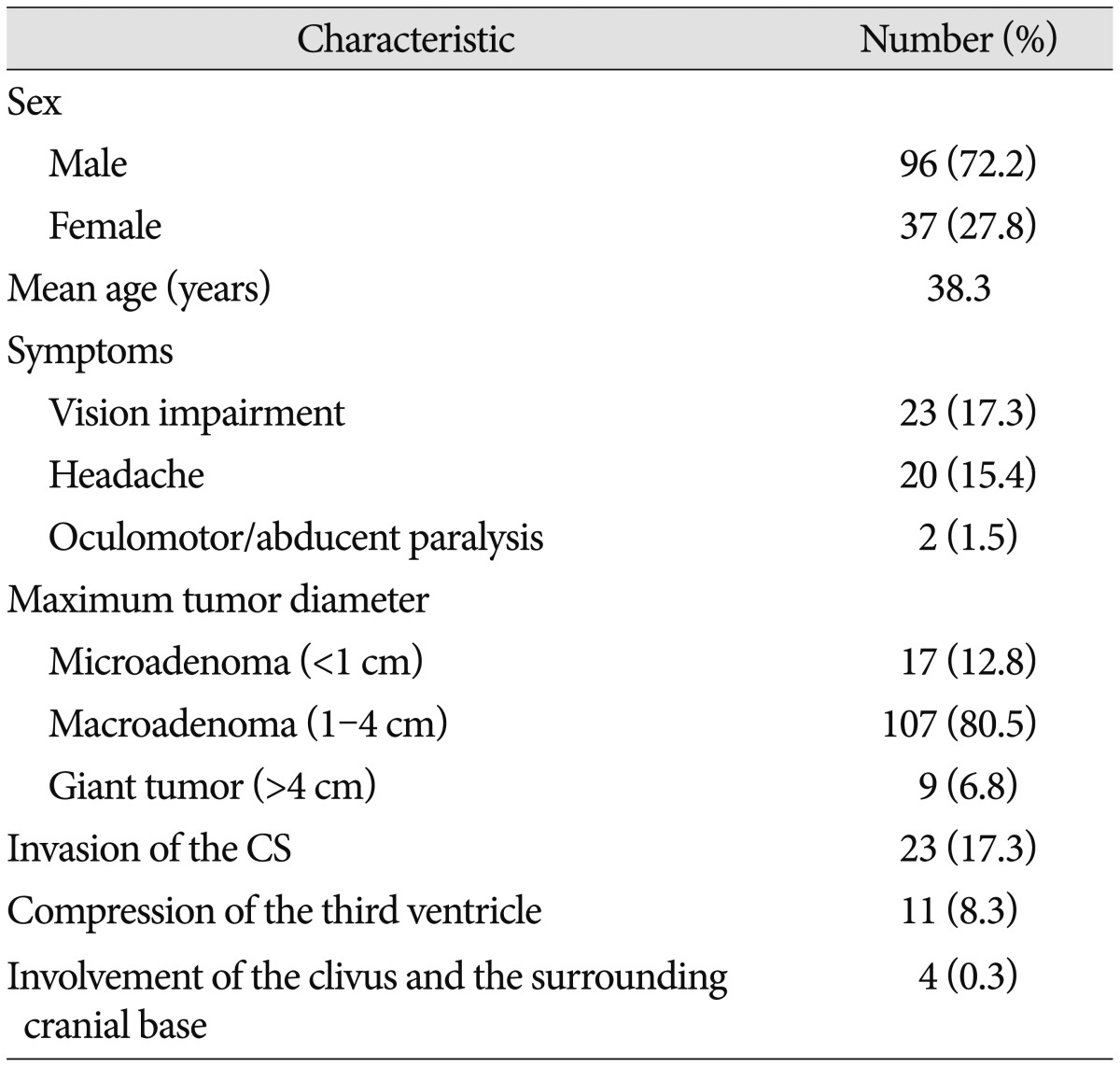
As shown in Table 1, a total of 133 patients were enrolled in this study, 96 male and 37 female, mean age 38.3 years (range, 10-67). The most common symptoms included vision impairment (23 cases), headache (20 cases), oculomotor/abducent paralysis (2 cases) and hyperglycemia (31 cases). Follow-up was carried out for 3-48 months. Microadenoma (<1 cm) was found in 17 cases, macroadenoma (1-4 cm) in 107 cases and giant tumor (>4 cm) in 9 cases. A total of 11 (8.3%) patients suffered from compression of the third ventricle. Evidence of cavernous sinus invasion according to the Knosp classification was collected from coronal T1-weighted contrasted imaging. Invasion of the cavernous sinus occurred in 23 (17.3%) cases, in which 16 patients had tumor crossing the medial intercarotic line, and the rest seven crossing the lateral line. Involvement of the clivus and the surrounding cranial base was seen in four (0.3%) cases. Preoperative endocrine tests revealed that all the 133 patients had elevated GH and IGF-1, 13 experienced hyperprolactinemia and five suffered hypopituitarism.
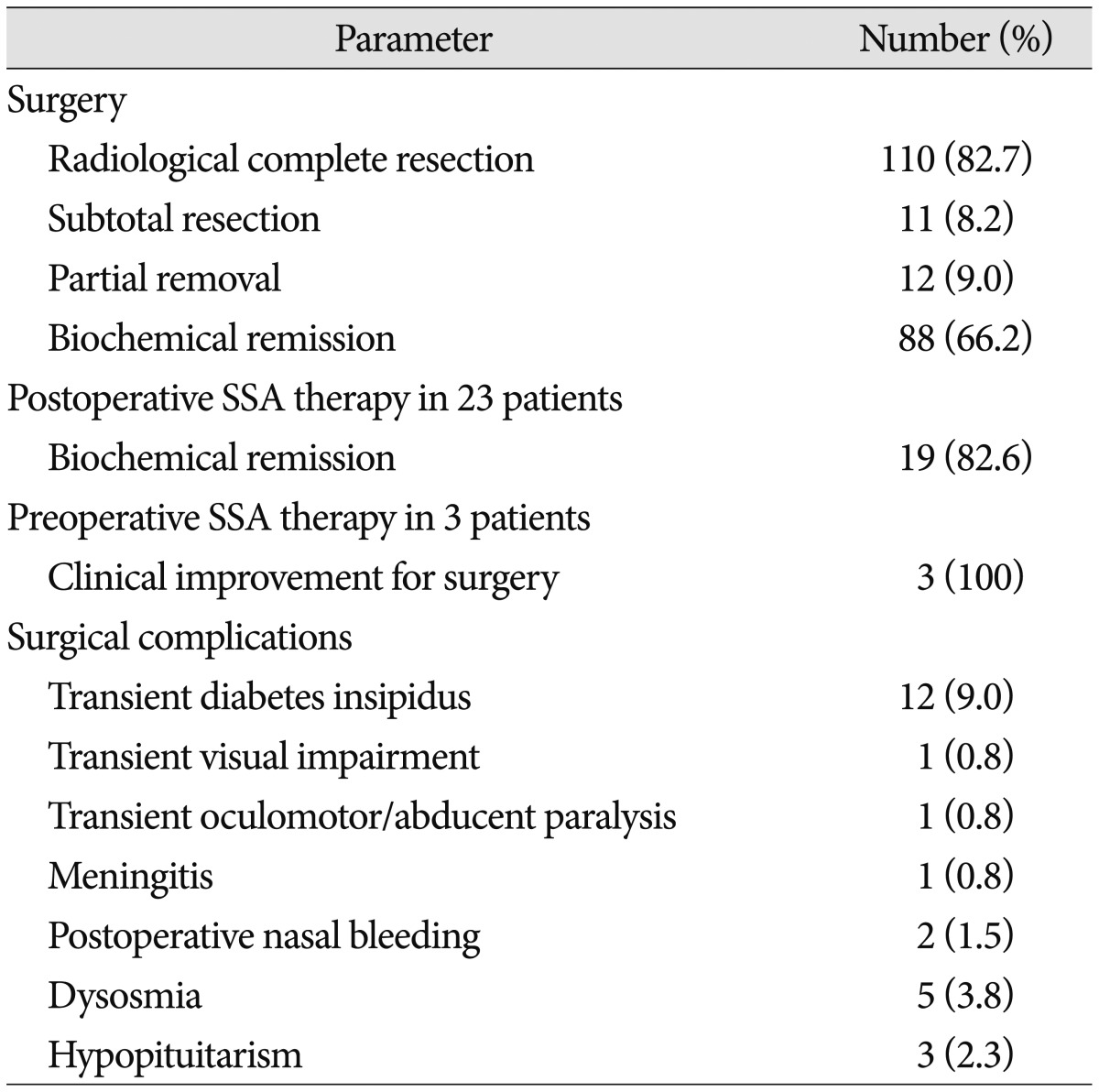
A total of 110 (82.7%) patients achieved radiological complete resection, 11 (8.2%) subtotal resection and 12 (9.0%) partial resection. However, only 88 (66.2%) patients showed biochemical remission, that was random GH level less than 2.5 µg/L, nadir GH level less than 1 µg/L after oral glucose administration and IGF-1 lower than age-corrected reference. Twenty-two out of the 23 patients who previously suffered from visual defect achieved improvement. Thirty-three patients showed suspected cavernous sinus and cranial base involvements on preoperative MRI, of which 23 (69.7%) achieved radiological complete removal, 13 (39.4%) reached nadir GH less than 1 ng/mL. Seven patients showed tumor crossing the lateral intercarotid line on preoperative MRI, of which only one achieved total resection and none achieved biochemical remission (Table 2).
No mortality or severe disability was observed during follow up. One patient had transient visual impairment and recovered before discharge. One patient had transient oculomotor/abducent paralysis and recovered within one month after surgery. Surgical repair with artificial dura matter was successfully performed in 19 (14.3%) cases with or potential with cerebrospinal leak during surgery. Preventive post-surgery lumbar cerebrospinal fluid drainage was adopted in five cases. None of the patients required a second time repair surgery for post-operative CSF leak. One patient suffered from meningitis, which was cured without any neurological sequela. Postoperative nasal bleeding occurred in two patients and one patient received embolotherapy via external carotid route. Twelve (9.0%) patients experienced transient diabetes insipidus and five (3.8%) patients had dysosmia within three months after surgery. Three patients experienced hypopituitarism and required thyroid hormone as well as steroid replacement. Five patients had hypogonadism and required no medication (Table 2).
Neuro-navigation was performed in 51 (38.3%) cases with complicated tumors, no operation delay or tissue injury when searching sellar floor and tumor. Out of 15 patients who applied iMRI, four received reoperation because of positive findings on iMRI and the tumors were not removable because of their locations such as cavernous sinus. We firstly adopted a high field st-rength iMRI to guide endoscopic transsphenoidal surgery in China and found one case with residual tumor stroke, so that a second operation was avoided.
Preoperative long-acting SSA was given to three patients who were illegible for operation due to heart failure and uncontrolled hyperglycemia. After 1-3 months of SSA therapy, improvement was observed in both left ventricle ejection fraction (LVEF) and blood glucose. All the three patients successfully went through the operation. Long-acting SSA (20 mg every 30 days) was administered to 23 patients who showed elevated GH level (23.5±7.5 µg/L) after surgery, with or without residual tumor on postoperative MRI. Nadir GH level less than 1 ng/mL was achieved in 19 (82.6%) patients including nine who had intermittent SSA therapy due to cost issues.
In this study, we performed a retrospective study in cohort of 133 patients with GH producing pituitary adenoma who were operated by a single surgeon in a single institute. Follow-up studies found 90.9% with radiological complete or subtotal resection and 66.2% with biochemical remission. SSA further achieved biochemical remission in 82.6% patients with persistently elevated GH level after surgery.
The goals of treating GH producing adenoma are to relieve symptoms, control tumor growth, achieve biochemical control or cure, save pituitary function, reduce mortality and morbidity2,8,11). A meta-analysis confirmed that normalizing hormone in acromegaly patients reduced mortality rate as to normal population6).
Transsphenoidal surgery is considered the first line therapy for GH-secreting tumors. It is associated with excellent safety profile (morbidity <2%; mortality <0.1%) in experienced medical centers5,7). Moreover, by surgical resection the cure rate of pituitary microadenoma is approximately 80% and macroadenoma 50%. Recently, surgery strategies have witnessed significant technique improvement, including neuro-navigation and intraoperative MRI as well as endoscopy4,15,17). The application of these new technologies further improves the safety and efficacy of transsphenoidal surgery. The advantages of transnasal endoscopic procedures over the traditional transnasal microscopic surgery included lower morbidity, greater satisfaction and shorter hospitalization but without compromising surgical success when performed by experienced surgeons. Endoscopic surgery has been recommended by international societies as therapy options2,8,10). No doubt that treatment in accordance with clinical guidelines by experienced hands at specialized medical center ensures the safety and effectiveness. This had already been confirmed by a UK study3). In our cohort, we found similar rate of safety and efficacy as those from experienced institute. Neuro-navigation was performed in 51 (38.3%) cases with complicated tumors, no operation delay or tissue injury when searching sellar floor and tumor. Out of 15 patients who applied iMRI, four received reoperation and achieved complete resection. Intraoperative MRI helps discovering the residual tumor and can guide resection. Nonetheless, not all the residual tumor is resectable, for example those located in the cavernous sinus or far away from the sellar region. However, as to the invasive GH-secreting tumors, the surgery cure rate is significant lower than that of non-invasive tumors. In patients with tumors growing sideways into the cavernous sinus, it was difficult to completely remove the tumor without causing injury to the nerves and vessels, and hormonal control is almost impossible by surgery alone.
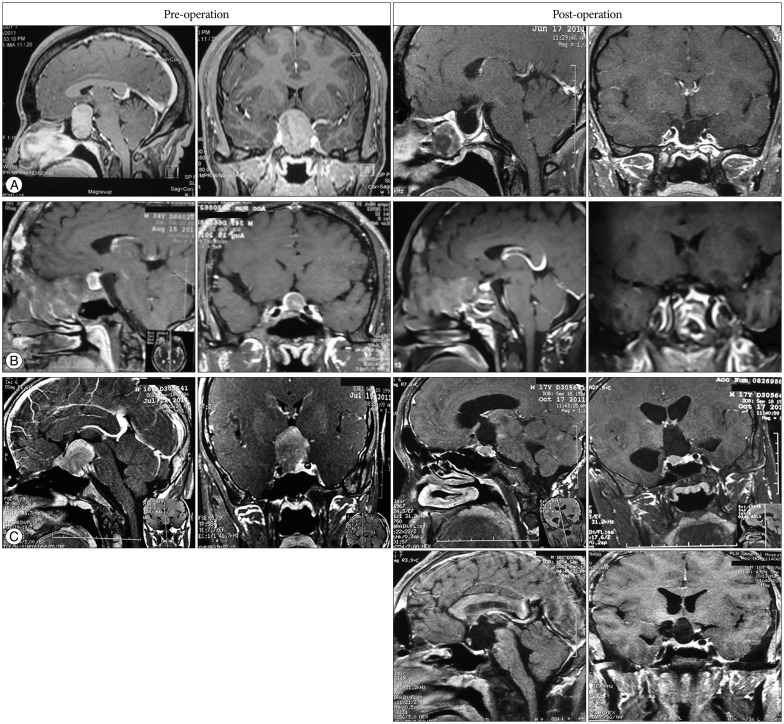
The introduction of long-acting SSA provides an alternative to surgery. It was reported that as many as 60-70% of patients show-ed reduced GH to <0.094 nmol/L and 50-80% reached normalization of IGF-1 to age- and sex-matched levels; meanwhile, the tumor shrank especially in SSA-naive patients13). Therefore, SSA has been recommended by ACG, AACE, and China local guideline as the first-line therapy in certain patient groups : patients who are at high surgical risk, those with tumors that exhibit extrasellar involvement (without chiasmal compression) and low likelihood of cure with surgery, and those who express a preference for medical management. In present study, long-acting SSA was administered to 23 patients who showed elevated GH level after surgery, with or without residual tumor on postoperative MRI. Biochemical control was achieved in 19 (82.6%) patients. As shown in Fig. 1, with the development of endoscopy, iMRI and neuro-navigation system, large tumor, even anatomy unreachable located, can be totally removed and achieve biochemical cure (Fig. 1A, B); however, biochemical cure of some patients required additional SSA therapy after radiological complete resection has achieved (Fig. 1C). Although preoperative SSA reduced surgical risk and improve biochemical outcome, more studies are needed to support this statement12). We found SSA could efficiently optimize perioperative management by improving heart function and blood glucose level, which is consistent with other published data9,16). The cost of SSA is one limitation that restricts its wide application. Interestingly, we found intermittent SSA treatment might be a compromise option for those who cannot afford fulltime medication.
In summary, we found endoscopic transsphenoidal surgery achieved radiological and biochemical remission in 82.7% and 66.2% patients with GH producing pituitary adenoma. We suggest pre-surgery use of long-acting SSA to improve heart function and reverse metabolic disorders. For patients who failed to achieve biochemical remission, SSA is certainly the drug of choice. The limitation of this study was its retrospective design and short time of follow up.
Acknowledgements
The authors declare that they accepted the sponsorship of editorial support from Novartis China.
References
1. Barker FG 2nd, Klibanski A, Swearingen B. Transsphenoidal surgery for pituitary tumors in the United States, 1996-2000 : mortality, morbidity, and the effects of hospital and surgeon volume. J Clin Endocrinol Metab. 2003; 88:4709–4719. PMID: 14557445.

2. Colao A, Martino E, Cappabianca P, Cozzi R, Scanarini M, Ghigo E. A.L.I.C.E. Study Group. First-line therapy of acromegaly : a statement of the A.L.I.C.E. (Acromegaly primary medical treatment Learning and Improvement with Continuous Medical Education) Study Group. J Endocrinol Invest. 2006; 29:1017–1020. PMID: 17259801.

3. Gittoes NJ, Sheppard MC, Johnson AP, Stewart PM. Outcome of surgery for acromegaly--the experience of a dedicated pituitary surgeon. QJM. 1999; 92:741–745. PMID: 10581337.

4. Hlavica M, Bellut D, Lemm D, Schmid C, Bernays RL. Impact of ultra-low-field intraoperative magnetic resonance imaging on extent of resection and frequency of tumor recurrence in 104 surgically treated nonfunctioning pituitary adenomas. World Neurosurg. 2013; 79:99–109. PMID: 23043996.

5. Hofstetter CP, Mannaa RH, Mubita L, Anand VK, Kennedy JW, Dehdashti AR, et al. Endoscopic endonasal transsphenoidal surgery for growth hormone-secreting pituitary adenomas. Neurosurg Focus. 2010; 29:E6. PMID: 20887131.

6. Holdaway IM, Bolland MJ, Gamble GD. A meta-analysis of the effect of lowering serum levels of GH and IGF-I on mortality in acromegaly. Eur J Endocrinol. 2008; 159:89–95. PMID: 18524797.

7. Jane JA Jr, Starke RM, Elzoghby MA, Reames DL, Payne SC, Thorner MO, et al. Endoscopic transsphenoidal surgery for acromegaly : remission using modern criteria, complications, and predictors of outcome. J Clin Endocrinol Metab. 2011; 96:2732–2740. PMID: 21715544.

8. Katznelson L, Atkinson JL, Cook DM, Ezzat SZ, Hamrahian AH, Miller KK. American Association of Clinical Endocrinologists. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the diagnosis and treatment of acromegaly--2011 update. Endocr Pract. 2011; 17(Suppl):1–44. PMID: 21846616.

9. Maison P, Tropeano AI, Macquin-Mavier I, Giustina A, Chanson P. Impact of somatostatin analogs on the heart in acromegaly : a metaanalysis. J Clin Endocrinol Metab. 2007; 92:1743–1747. PMID: 17311857.

10. Melmed S, Colao A, Barkan A, Molitch M, Grossman AB, Kleinberg D, et al. Guidelines for acromegaly management : an update. J Clin Endocrinol Metab. 2009; 94:1509–1517. PMID: 19208732.
11. Nomikos P, Buchfelder M, Fahlbusch R. The outcome of surgery in 668 patients with acromegaly using current criteria of biochemical 'cure'. Eur J Endocrinol. 2005; 152:379–387. PMID: 15757854.

12. Pita-Gutierrez F, Pertega-Diaz S, Pita-Fernandez S, Pena L, Lugo G, Sangiao-Alvarellos S, et al. Place of preoperative treatment of acromegaly with somatostatin analog on surgical outcome : a systematic review and meta-analysis. PLoS One. 2013; 8:e61523. PMID: 23634209.
13. Plöckinger U, Quabbe HJ. Presurgical octreotide treatment in acromegaly : no improvement of final growth hormone (GH) concentration and pituitary function. A long-term case-control study. Acta Neurochir (Wien). 2005; 147:485–493. discussion 493. PMID: 15806331.

14. Rajasoorya C, Holdaway IM, Wrightson P, Scott DJ, Ibbertson HK. Determinants of clinical outcome and survival in acromegaly. Clin Endocrinol (Oxf). 1994; 41:95–102. PMID: 8050136.

15. Schwartz TH, Stieg PE, Anand VK. Endoscopic transsphenoidal pituitary surgery with intraoperative magnetic resonance imaging. Neurosurgery. 2006; 58(1 Suppl):ONS44–ONS51. discussion ONS44-ONS51. PMID: 16479628.

16. Shen M, Shou X, Wang Y, Zhang Z, Wu J, Mao Y, et al. Effect of presurgical long-acting octreotide treatment in acromegaly patients with invasive pituitary macroadenomas : a prospective randomized study. Endocr J. 2010; 57:1035–1044. PMID: 21099129.

17. Wu JS, Shou XF, Yao CJ, Wang YF, Zhuang DX, Mao Y, et al. Transsphenoidal pituitary macroadenomas resection guided by PoleStar N20 low-field intraoperative magnetic resonance imaging : comparison with early postoperative high-field magnetic resonance imaging. Neurosurgery. 2009; 65:63–70. discussion 70-71. PMID: 19574826.
Fig. 1
Pituitary MRI before and after treatment. A : Case 1. 28 yr, female, before surgery GH >40 µg/L, after surgery GH 0.40 µg/L. B : Case 2. 34 yr, male, Albright Syndrome, tumor was surrounded fibrous dysplasia of bone, before surgery GH >40 µg/L, after surgery GH 0.72 µg/L. C : Case 3. 16 yr, male, July 15, 2011, before craniotomy surgery, tumor located at suprasellar and intrasellar, extending to skull base, GH >40 µg/L; October 17, 2011, before trans-sphenoidal surgery, the residual tumor located at right intrasellar and pressed on right cavernous sinus, GH >40 µg/L; follow-up in 2013, no residual tumor supersellar and intrasellar, GH >40 µg/L. Then after treatment with long acting SSA for one year, the patient showed normal GH and improved symptoms including headache. GH : growth hormone, SSA : somatostatin analogue.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download