Abstract
Objective
Blood blister-like aneurysms (BBAs) resemble arterial dissections. The purpose of this study was to investigate the relationship between these two disease entities and highlight commonalities and distinct features.
Methods
Among 871 consecutive patients with aneurysmal subarachnoid hemorrhage, 11 BBAs of internal carotid artery and seven vertebral artery dissections (VADs) with a short segmental eccentric dilatation (Mizutani type 4), which is morphologically similar to a BBA, were selected. The following clinical factors were studied in each group : age, gender, risk factors, Hunt and Hess grade (HHG), Fisher grade (FG), vasospasms, hydrocephalus, perioperative rebleeding rate, and treatment outcome.
Results
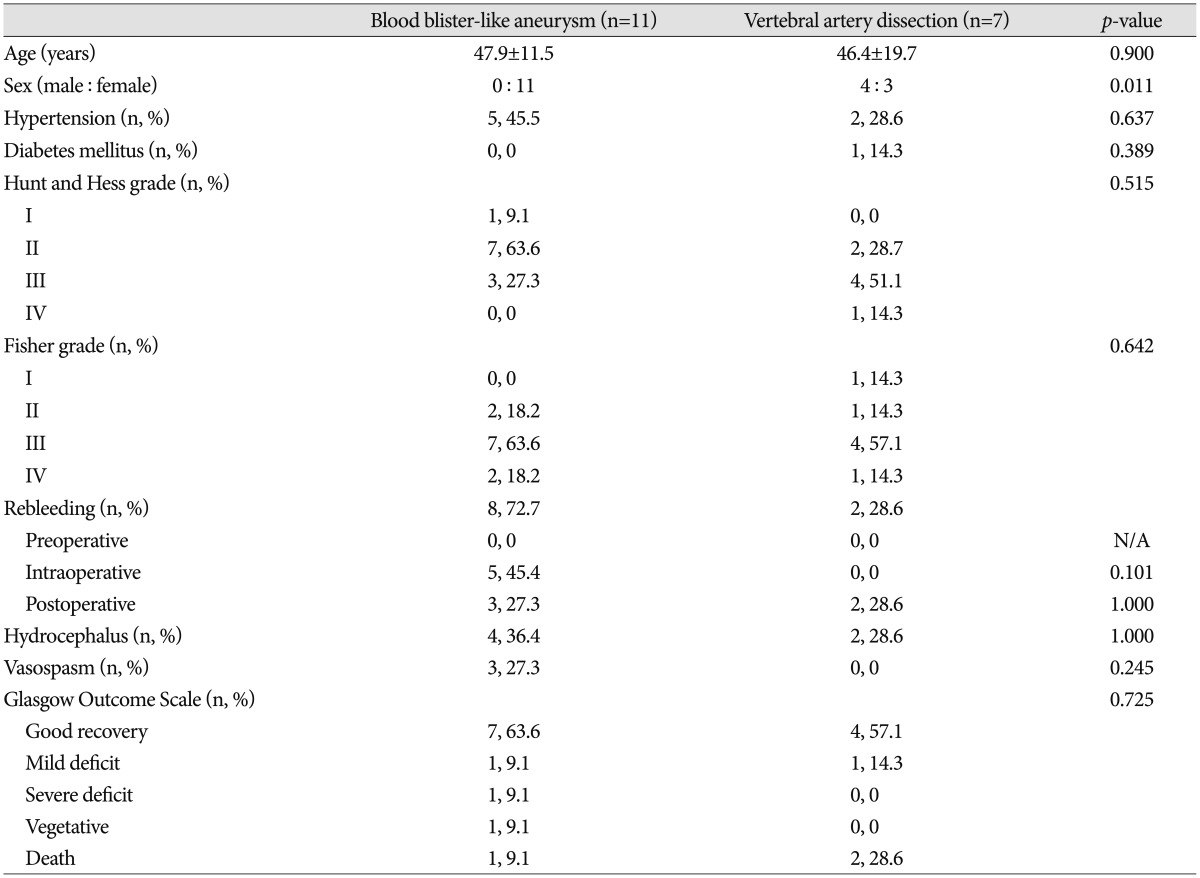
The mean age was 47.9 years in the BBAs group and 46.4 years in the type 4 VADs group. All the BBA patients were female, whereas there was a slight male predominance in the type 4 VAD group (male : female ratio of 4 : 3). In the BBA and type 4 VAD groups that underwent less aggressive treatment to save the parent artery, 29% (n=2/7) and 66.6% (n=2/3), respectively, eventually required retreatment. Perioperative rebleeding occurred in 72.7% (n=8) and 28.6% (n=2) of patients in the BBA and type 4 VAD groups, respectively. There was no statistical difference in the other clinical factors in both groups, except for the male dominancy in the type 4 VAD group (p=0.011).
Conclusion
BBAs and ruptured type 4 VADs have a similar morphological appearance but there is a distinct clinical feature in gender and perioperative rebleeding rates. Complete isolation of an aneurysm from the parent artery might be the most important discipline for the treatment of these diseases.
Blood blister-like aneurysms (BBAs) have several unique characteristics that differ from those of regular saccular aneurysms. These include 1) a typical small hemispheric, broad-based appearance, with a lack of an identifiable neck originating from a non branching site of an artery1,10,15,16,22); 2) instability, with the lesion showing morphological changes on short-term angiographic follow up1,15,16,22); and 3) fragility, with the lesion prone to rupture or regrowth after incomplete treatment1,10,15,16,22). Interestingly, these three typical properties of BBAs (appearance, instability, and fragility) seem to be observed in vertebral artery 395dissections (VADs)2,7,13,17,21). Some authors have suggested that a BBA is a kind of focal dissection or a lesion related to dissection4,12,15,16,19,20). Of interest, BBAs are renowned for their unique clinical homogeneity, whereas VADs are known for their diverse clinical presentation, including their location (extracranial vs. intracranial), initial presenting symptom (hemorrhage vs. ischemia), and prognosis, which depends on the location, shape, and symptoms3,5,17,21). A specific dissection group, such as hemorrhagic intracranial Mizutani type 4 VADs, usually have similar radiologic appearance with BBAs such as small hemispheric, broad-based dome arising at a non branching artery. In the present study, we selected a consecutive series of patients with ruptured intracranial VADs with Mizutani type 4 and compared their demographic, radiological, and clinical data with those of BBAs to elucidate common and distinct features of these two disease entities.
Over a 6-year period, 871 patients with aneurysmal subarachnoid hemorrhage (SAH) were treated at a tertiary referral center. Fourteen of these patients had aneurysms along the anterior aspect of the nonbranching sites of the internal carotid artery (ICA). Among these, three cases of saccular aneurysm were excluded and the remaining 11 cases of BBAs with typical angiographic findings of a broad-based, small hemispheric appearance1,15,16) were included in this study. All the patients were intraoperatively confirmed as having a BBA, except one patient in whom endovascular treatment was attempted. During the same period, 64 cases of spontaneous VADs in 58 patients were treated at the same institute. Four patients had extracranial (one in V2, three in V3) VAD and these patients presented with non-hemorrhagic symptoms. The other 54 patients with intracranial VAD showed various clinical presentations. Of these, 20 patients had SAH due to a rupture VAD. Among 20 ruptured intracranial VADs, seven patients had VADs with a short segmental eccentric dilatation (Mizutani type 4) which is morphologically similar to a BBA.
The clinical and radiological data of the 11 patients with BBAs and the seven patients with ruptured intracranial VADs with Mizutani type 4 were retrospectively reviewed. The variables assessed included age, gender, vascular risk factors (e.g., hypertension and diabetes mellitus), Hunt Hess grade9), Fisher grade6), hydrocephalus, vasospasms, and clinical outcome (preoperative, intraoperative, and postoperative rebleeding and the Glasgow Outcome Scale11) at the time of discharge). Independent t-tests, Mann-Whitney tests, and Fisher's exact test were performed for the statistical analysis using SPSS version 15 (SPSS Inc., Chicago, IL, USA). A p value less than 0.05 was considered statistically significant.
The mean age of the patients in the BBA and Mizutani type 4 VAD group was 47.9±11.5 and 46.4±19.7, respectively. All the patients in the BBA group were women, whereas there was a slight predominance of males in the VAD group (male : female ratio of 4 : 3, p=0.011). Five of the 11 (45.5%) patients with BBAs had a history of hypertension, and two of the seven (28.6%) patients in the VAD group had a history of hypertension. The prevalence of diabetes mellitus was slightly higher in the VAD group (1 of 7 cases, 14.3%) than in the BBA group (none of the 11 cases). The initial Hunt and Hess grades and Fisher grades on admission were not significantly different between the groups (Table 1).
Ten of the BBAs were treated with microvascular surgery, and one was treated with endovascular occlusion. Among those who underwent microsurgery, seven BBAs were clipped, and three were treated with clip wrapping. The three BBAs treated with clip wrapping were completely secured from the circulation. However, rebleeding occurred in two of the seven (28.6%) BBAs treated with clipping. One of these required re-clipping with ICA stenosis, and one required trapping of the ICA. One patient treated with endovascular trapping of the ICA rebled. This patient expired, despite nearly complete occlusion of the parent artery.
Various treatment modalities, such as endovascular trapping (n=4), stenting (n=2), and simple coiling (n=1), were attempted in the VAD group. Excellent angiographic results were achieved in all the cases treated with trapping and in one case of stenting. Additional therapeutic occlusion such as endovascular internal trapping and stent-assisted coil embolization of the vertebral artery was needed in one case of stenting and in one case of simple coiling, respectively, due to rebleeding and regrowth of the pseudoaneurysm.
There was no statistically significant between-group difference in preoperative and postoperative rebleeding. Interestingly, the postoperative rebleeding rate was high in both groups (n=3, 27.3% in BBA; n=2, 28.6% in VAD) compared to that of usual saccular aneurysms.14) Intraoperative rebleeding occurred more frequently in the BBA group (n=5, 45.5%) than in the VAD group (n=0, 0%) although statistical significance was not found. Symptomatic vasospasms developed in three (27.3%) of the patients with BBAs and in none of the patients with VADs (p=0.245). There was no statistically significant between-group difference in the development of hydrocephalus or in the Glasgow outcome scale at the time of discharge.
There are limited data in the literature related to the pathomechanism underlying the development of BBAs. One proposed pathomechanism of BBA is short segment arterial dissection4,12,15,16,19,20). Mizutani et al.12) classified BBAs as a subtype of nonatherosclerotic dissecting aneurysm. Day et al.4) speculated that a BBA represents a focal dissection, resembling that seen in the proximal intracranial vertebral artery near the origin of the posteroinferior cerebellar artery. Not only has the hypothesis been reported that BBA might be a kind of dissection15,20), but also the association with ICA dissection as a causative factor for BBA has been reported16,19).
Ishikawa et al.10) provided a valuable pathological description of a BBA that included disruption of the internal elastic lamina and media, which were covered with normal adventitia. Interestingly, aformentioned findings seem very similar to those of pseudoaneurysms in arterial dissection8).
Nevertheless, it is not easy to correlate the natural history of a BBA with that of an arterial dissection arising in other parts of the vasculature in the clinical setting. This is mainly because the clinical features (shape, location, clinical presentation, and prognosis) of arterial dissections are more variable than those of BBAs which are strikingly homogeneous3,5,17,21,24). However, intracranial ruptured arterial dissections have surprisingly similar clinical characteristics in terms of appearance, fragility, and instability to those of BBAs2,7,13,17,21,24). In a ruptured VAD, Friedman and Drake7) described operative findings of a "blister-thin" arterial wall. Many pseudoaneurysms of the intracranial VADs angiographically show focal aneurysmal dilatation21), without a definite neck (Fig. 1), as is seen in BBAs22). BBAs and VADs appear to have unique characteristics for their anatomic location, as both of them are rarely seen at arterial bifurcations. In addition to the dynamic nature of VADs, which include morphological changes on serial angiographic follow up (e.g., recanalization or pseudoaneurysm formation)2,7,13,24), incomplete treatment of a ruptured VAD usually results in dramatic aneurysmal growth or rupture2,7,17,24), and all of this represents their instability.
We assumed that the similarity of VADs and BBAs may be due to their similar anatomical origin, with both usually developing in relatively large, nonbranching vessels. These vessels have an abrupt curvature and run from the fixed segment in fibrous tissue to the straightened movable segment in the wide cisternal space. The relatively large-bore, straightened vessels just behind the acute curvature might be more susceptible to hemodynamic stress.
One major difference between VADs and BBAs is the occasional presence of an intimal flap in the former that is hardly ever found in the latter. The short segment dissection in VAD shows typical aneurysmal dilatation, without an intimal flap or pseudolumen18,21). In contrast, VAD involving the long vessel segment is often accompanied by an intimal flap, a true/false lumen, the pearl and string sign, and intraluminal thrombosis or recanalization3,21). From a similar point of view, it is conceivably assumed that short segment involvement of BBA might be attributed to the absence of the intimal flap.
In this study, the patients in each group were well matched for age, vascular risk factors, clinical grade, and radiological grade, although the patients were consecutively enrolled. In accordance with previous studies on VADs, the patients in both groups were relatively young3,5,13,18,21). When compared BBAs and Mizutani type 4 VADs, many clinical features of them showed very similar pattern in addition to their common radiologic features. Gender and intraoperative rebleeding were different between the two groups. Many other studies have established the female dominancy in BBAs, although the reason for this is uncertain16,20,22). They have also reported that VAD shows a male preponderance18,21,23).
The overwhelming majority of BBAs showed intraoperative rupture. This is because most of the BBAs were treated with microsurgery in which direct manipulation of the fragile aneurysmal dome is frequently attempted, in contrast to most of the VADs, which were mainly treated with an endovascular method. Postoperative rebleeding was much more frequent in both groups than in patients with other saccular aneurysms. Thus, successful treatment in both BBAs and VADs can be guaranteed only with complete separation of the diseased lesion from the normal circulation. Postoperative rebleeding seems to be a common characteristic of both BBAs and VADs. In contrast, recanalization, regrowth, compaction, and a neck remnant after clipping or coiling of other saccular aneurysms do not usually result in rebleeding14).
These findings in this article do not necessarily point to these two disease entities having a similar natural history nor having different clinical features. However, they may provide some clues as to the pathomechanism underlying these rare and interesting aneurysms and lead to a large-scale study in the future.
Mainly due to difficulty obtaining pathological specimens of BBA and VAD, we could not compare pathological differences between the two groups. This study cannot draw definitive conclusions due to the lack of a thorough analysis of 3D angiography, including a study of flow dynamics and an assessment of the vessel wall by repeated imaging, in addition to an analysis of blood parameters, coagulation in particular. However, we rather insist on the severity of both disease, and on the poor outcomes of the disease in the majority of the cases whatever the treatment is applied, bringing into the light the fact that trapping seems to be better than other less invasive therapeutic options in both conditions.
The various presentations of VAD (clinically and radiographically) as well as the different proportions of males and females in the two groups, should be the subjects of further studies. In addition, the reason for a long segment and bilateral involvement of VAD (which almost exclusively does not happen in BBA) should be clarified in the future. Further pathological study is needed to elucidate the pathophysiological mechanisms for these disease entities.
After excluding the differences in the treatment modalities (microsurgery or endovascular surgery), as well as the location (anterior circulation or posterior circulation), similar clinical characteristics of BBAs and ruptured Mizutani type 4 VADs are that they occur in young patients and have unstable hemodynamics. However, there was a significant difference in gender and intraoperative rebleeding rates between the groups. The need to completely isolate the aneurysm from the parent artery might be the most important thing in common between these two disease entities.
References
1. Abe M, Tabuchi K, Yokoyama H, Uchino A. Blood blisterlike aneurysms of the internal carotid artery. J Neurosurg. 1998; 89:419–424. PMID: 9724116.

2. Anxionnat R, de Melo Neto JF, Bracard S, Lacour JC, Pinelli C, Civit T, et al. Treatment of hemorrhagic intracranial dissections. Neurosurgery. 2008; 62(6 Suppl 3):1525–1531. PMID: 18695573.

3. Arnold M, Bousser MG, Fahrni G, Fischer U, Georgiadis D, Gandjour J, et al. Vertebral artery dissection : presenting findings and predictors of outcome. Stroke. 2006; 37:2499–2503. PMID: 16960096.
4. Day AL, Gaposchkin CG, Yu CJ, Rivet DJ, Dacey RG Jr. Spontaneous fusiform middle cerebral artery aneurysms : characteristics and a proposed mechanism of formation. J Neurosurg. 2003; 99:228–240. PMID: 12924694.

5. de Bray JM, Penisson-Besnier I, Dubas F, Emile J. Extracranial and intracranial vertebrobasilar dissections : diagnosis and prognosis. J Neurol Neurosurg Psychiatry. 1997; 63:46–51. PMID: 9221967.

6. Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. 1980; 6:1–9. PMID: 7354892.

7. Friedman AH, Drake CG. Subarachnoid hemorrhage from intracranial dissecting aneurysm. J Neurosurg. 1984; 60:325–334. PMID: 6693960.

8. Hamada Y, Mannoji H, Kaneko Y. A ruptured dissecting aneurysm of the vertebral artery : comparison of angiographic and histological findings. Neuroradiology. 2001; 43:375–378. PMID: 11396741.

9. Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg. 1968; 28:14–20. PMID: 5635959.

10. Ishikawa T, Nakamura N, Houkin K, Nomura M. Pathological consideration of a "blister-like" aneurysm at the superior wall of the internal carotid artery : case report. Neurosurgery. 1997; 40:403–405. discussion 405-406. PMID: 9007879.

11. Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975; 1:480–484. PMID: 46957.
12. Mizutani T, Miki Y, Kojima H, Suzuki H. Proposed classification of nonatherosclerotic cerebral fusiform and dissecting aneurysms. Neurosurgery. 1999; 45:253–259. discussion 259-260. PMID: 10449069.

13. Mokri B, Houser OW, Sandok BA, Piepgras DG. Spontaneous dissections of the vertebral arteries. Neurology. 1988; 38:880–885. PMID: 3368069.

14. Molyneux AJ, Kerr RS, Yu LM, Clarke M, Sneade M, Yarnold JA, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms : a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005; 366:809–817. PMID: 16139655.

15. Nakagawa F, Kobayashi S, Takemae T, Sugita K. Aneurysms protruding from the dorsal wall of the internal carotid artery. J Neurosurg. 1986; 65:303–308. PMID: 3734880.

16. Ogawa A, Suzuki M, Ogasawara K. Aneurysms at nonbranching sites in the surpaclinoid portion of the internal carotid artery : internal carotid artery trunk aneurysms. Neurosurgery. 2000; 47:578–583. discussion 583-586. PMID: 10981744.

17. Park SI, Kim BM, Kim DI, Shin YS, Suh SH, Chung EC, et al. Clinical and angiographic follow-up of stent-only therapy for acute intracranial vertebrobasilar dissecting aneurysms. AJNR Am J Neuroradiol. 2009; 30:1351–1356. PMID: 19342544.

18. Ro A, Kageyama N, Abe N, Takatsu A, Fukunaga T. Intracranial vertebral artery dissection resulting in fatal subarachnoid hemorrhage : clinical and histopathological investigations from a medicolegal perspective. J Neurosurg. 2009; 110:948–954. PMID: 19199507.

19. Satoh A, Nakamura H, Odaki M, Kobayashi S, Kageyama Y, Fukuda K, et al. High risk aneurysms of the internal carotid artery : dorsal IC aneurysm. Surg Cereb Stroke. 1993; 21:467–472.
20. Shigeta H, Kyoshima K, Nakagawa F, Kobayashi S. Dorsal internal carotid artery aneurysms with special reference to angiographic presentation and surgical management. Acta Neurochir (Wien). 1992; 119:42–48. PMID: 1481751.

21. Shin JH, Suh DC, Choi CG, Leei HK. Vertebral artery dissection : spectrum of imaging findings with emphasis on angiography and correlation with clinical presentation. Radiographics. 2000; 20:1687–1696. PMID: 11112824.

22. Sim SY, Shin YS, Cho KG, Kim SY, Kim SH, Ahn YH, et al. Blood blister-like aneurysms at nonbranching sites of the internal carotid artery. J Neurosurg. 2006; 105:400–405. PMID: 16961134.

23. Yamaura A, Watanabe Y, Saeki N. Dissecting aneurysms of the intracranial vertebral artery. J Neurosurg. 1990; 72:183–188. PMID: 2404089.

24. Yoon SM, Shim JJ, Kim SH, Chang JC. Bilateral vertebral artery dissecting aneurysms presenting with subarachnoid hemorrhage treated by staged coil trapping and covered stents graft. J Korean Neurosurg Soc. 2012; 51:155–159. PMID: 22639713.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download