Abstract
Objective
To assess the effect of bone marrow mononuclear cells (BMMNCs) transplantation in the expression of nuclear factor-κB (NF-κB) in spinal cord injury (SCI) in rats.
Methods
BMMNCs were isolated from tibia and femur by a density gradient centrifugation. After establishment of acute transection SCI, rats were divided into experiment (BMMNCs), experiment control (0.1 M PBS infused) and sham surgery groups (laminectomy without any SCI). Locomotor function was assessed weekly for 5 weeks post-injury using BBB locomotor score and urinary bladder function daily for 4 weeks post-injury. Activity of NF-κB in spinal cord was assessed by immunohistochemistry and reverse transcriptase polymerase chain reaction.
Results
At each time point post-injury, sham surgery group had significantly higher Basso, Beattie, Bresnahan locomotor and urinary bladder function scores than experiment and experiment control group (p<0.05). At subsequent time interval there were gradual improvement in both experiment and experiment control group, but experiment group had higher score in comparison to experiment control group (p<0.05). Comparisons were also made for expression of activated NF-κB positive cells and level of NF-κB messenger RNA in spinal cord at various time points between the groups. Activated NF-κB immunoreactivity and level of NF-κB mRNA expression were significantly higher in control group in comparison to experiment and sham surgery group (p<0.05).
Spinal Cord Injury (SCI) refers to damage to spinal cord that is caused by trauma and results in paralysis and loss of sensation. Depending on location of the injury, SCI may lead to loss of movement, loss of sensation, loss of control on bowel and bladder habit, exaggerated reflex as well as pain31). With growing modernization, incidence of spinal cord injury has sharply risen up. Motor-vehicle accidents, falls, violence and sport injuries are the most common causes of SCI. High physical and psychological effects are imposed to individual as well as to family and society. SCI has become a cause of major clinical, social and economic problems. So, it is crucial to find the appropriate and ef-fective treatment strategies for cure and functional recovery after SCI.
The pathophysiology of SCI is complex and is characterized by 1) primary injury; resulting immediately from initial trauma and 2) Secondary injury; resulting as indirect effect from primary injury. Secondary Injury initially occurs within hours, days and weeks following primary injury. It initially occurs at the site of primary injury and then gradually spreads to adjacent uninjured tissue in the vicinity16). The most significant mechanisms underlying secondary injury are vascular disturbances, inflammation, lipid peroxidation, excitotoxicity, apoptosis, demyelination and ionic disturbances7). Neurons continue to die in the secondary injury phase, which is determined by large number of cellular, molecular and biochemical cascade. This phase of neuronal death is potentially an avoidable event10). So, it is important to understand the underlying molecular and cellular events in the secondary injury phase of SCI to prevent devastating outcome after SCI.
Inflammation, which is generally induced by SCI, is the main component of secondary injury and plays a vital role in regulating the pathogenesis of SCI that has deleterious effect on tissue recovery and aggravates progressive necrosis of the cells in damaged area. The extent of inflammatory response is increased by pro-inflammatory cytokines and chemokines, leading to apoptotic cell death. Range of tissue injury and consequent disability may be limited by attenuating early inflammatory response23). Nuclear factor-kappa B (NF-κB) is the principal transcription factor responsible in regulating gene expression that mediates inflammation1).
NF-κB factor is a protein complex, pleotropic in nature that controls the transcription of DNA. NF-κB consist dimers of five subunits P50/P105 (NF-κB1), P52/P100 (NF-κB2), C-Rel, P65/Rel A and Rel B. Each subunit of NF-κB/Rel family members participates in the formation of various homo- or heterodimers that are likely to confer a degree of target gene specificity. The prototypical NF-κB complex is a heterodimer that is composed of P50 and Rel A8). NF-κB is a ubiquitous transcription factor found in the cytoplasm and regulates a number of pathophysiological processes, such as inflammation, apoptosis, immune response as well as other genetic program that are central to cell growth and survival8,24). Inhibitory factor IκB complexes with NF-κB and retains it in inactivated state in cytosol. Protein kinase Inhibitory Factor κB Kinase complex (IKK) degrades IκB leading to release, activation and translocation of NF-κB to nucleus, where it's interaction with a specific DNA sequence in the promoter region leads to transcription of wide array of genes. IKK is a pivotal player for activation of NF-κB by most stimuli30).
Limitation of functional recovery in patient with SCI has led to investigations of various treatment strategies. Cell therapies have been considered as a promising treatment strategy. Recently, stem cells are being used as one of the possible approaches for repairing spinal cord after its injury28). Stem cells have self renewal and multi-potential properties and may replace damaged cells, provide neuro-protection or create environment favoring regeneration by endogenous cells, thus helping in repair of injured nervous tissue25). Bone marrow mononuclear cells (BMMNCs) are considered as appropriate stem cells that can be used in SCI. BMMNCs are multipotent progenitor cells obtained by taking heterogeneous cell population found in bone marrow36). BMMNCs consist of hematopoietic stem cells, mesenchymal stem cells and endothelial cells37). BMMNCs are attractive for transplantation because they can be easily obtained from bone marrow and transplanted back into original donor eliminating the risk of rejection.
Therefore, we investigated whether BMMNCs could down-regulate the expression of NF-κB and thus improve functional recovery in rats after SCI.
The present study was conducted at Central Experimental Laboratory of first affiliated hospital of Jiamusi University, China from April 2013 to August 2013.
A total of 80 female and 10 male Wister rats of specific pathogen free grade, aged 10-12 weeks, weighing 180-220 gm were provided by the animal experimental center of Jilin University, Changchun, China (SCXK-2011-004). The study was approved by the animal research ethic committee of Jiamusi University. All experimental procedures were performed in accordance with the guidance and suggestions for care and use of laboratory animals, published by the Ministry of Science and Technology of People's Republic of China34). Rats were maintained in temperature controlled room (21+/-2℃) on 12 hours light/dark cycle with rodent standard chow and water available ad libitum. Rats were acclimatized to their environment for 1 week before any experimental procedure.
Rats were divided into three groups : Sham surgery group (n= 15) : Rats with only laminectomy but without any SCI; Experiment control group (n=30) : Rats with acute transection SCI and treated with phosphate buffer solution (PBS) and Experimental group (n=35) : Rats with acute transection SCI and treated with BMMNCs.
Male rats were selected for isolation of BMMNCs as it has been reported that bone marrow of male rats contain more BMMNCs in comparison to female rats. Rats were anaesthetized with 10% chloral hydrate (300 mg/kg body weight) injected intraperitoneally. All the procedures were performed under strict aseptic technique. Both the femurs and tibias were extracted and epiphyses were removed. A 20 G needle was inserted intramedullary on the proximal end of each bone and flushed with 2 mL of Normal Saline mixed with 0.1% ethylenediaminetetraacetic acid (EDTA). Each bone was turned upside down and flushed again from distal end too. The collected suspension was centrifuged @1500 rpm for 10 minutes at 4℃. Supernatant was discarded and retained pellets were rinsed with 5 mL of 0.1 M PBS. The diluted PBS suspension was gently overlayed on 5 mL of Histopaque solution (Beijing Hao Yang Biological Engineering Company, Beijing, China), then subjected to density gradient centrifugation for isolation of BMMNCs, centrifuged @2000 rpm for 20 minutes at 4℃. BMMNCs layer seen as a distinct cloudy layer in about middle of solution was collected and rinsed with 5 mL of 0.1 M PBS and centrifuged again at 1000 rpm for 10 minutes at 4℃. Thus collected pellet cells were kept in Dulbecco's modified eagle medium (DMEM). Cell concentration was adjusted to 6×107/dL and stored in refrigerator until transplantation (up to 3 hours). The viability of BMMNCs was assessed using tryphan blue solution and found to be more than 90%.
Rats were anaesthetized by intra-peritoneal injection of 10% Chloral hydrate (300 mg/g body weight). Under all aseptic precautions and draping, approximately 15 mm long longitudinal mid-line incision was made over T9-T11 vertebral region exposing paravertebral muscles. These muscles were dissected away bilaterally. Laminectomy at T10 was done, spinal cord exposed and transected. 15 µL cell suspension was infused in the vertebral canal at rostral and caudal segment, 1 cm from injured site, via 0.20 mm epidural catheter and Hamilton syringe for experimental group; whereas 0.1 M PBS was used instead of cell suspension for experimental control group. Catheter was left in position for 5 minutes following the completion of infusion to prevent cell leakage through the catheter track. For Sham surgery group, only laminectomy at T10 without any SCI was performed. Paravertebral muscles and skin was sutured in layers. 1 mL of Normal saline (NS) with 0.1 mL crystalline penicillin (160000 units diluted in 2 mL NS) was injected subcutaneously following surgery in order to replace the body fluid loss during surgery and as prophylaxis for any post-operative infection. During recovery from anesthesia, rats were placed on warm pad and covered with warm towel until re-establishment of thermoregulation and righting reflex. After appearance of symptoms such as tail wagging reflex and paralysis of both hind limbs, the models were considered successful. Rats were nursed and housed in a cage with water and softened rodent chow provided ad libitum. During the experiment period, rats' bladder and bowel were evacuated manually twice a day until voluntary control over function was acquired. Antibiotic was injected subcutaneously for 7 days to prevent any infection.
Motor function recovery after SCI was assessed using open field walking test. Evaluations were performed by investigators in a double blind mode. Locomotor assessment was begun 1 week after injury and performed weekly for 5 weeks. During free locomotion on floor, each hind limb was graded individually according to Basso, Beattie, Bresnahan (BBB) locomotor score2).
Urinary bladder function was assessed daily after SCI. The assessment was based on the bladder function scoring system developed by Martin Schwab's Lab21). Urinary bladder function was assessed and scored as :
1) Dysfunction (a full bladder, medium to high pressure required for manual voiding of bladder)
2) Normal function (empty to half full bladder, voiding after slight touch)
After a respective allotted time (i.e., 6 hours, 1 day, 3 days, 7 days, and 14 days) after SCI, rats were euthanized with a lethal dose of 10% chloral hydrate (600 mg/kg body weight) injected intra-peritoneally. For rats undergoing reverse transcription polymerase chain reaction (RT-PCR) analysis, no cardiac perfusion was done. -20 mm long spinal cord with injured/laminectomy site as an epicenter was dissected out from the body and flash frozen at -80℃. For rats undergoing immunohistochemical analysis, intra-cardiac perfusion was performed using 200 mL Normal saline to wash the blood out of the circulatory system followed by 300 mL of 4% paraformaldehyde in 0.1 M PBS (pH 7.4). About 20 mm long spinal cord segment with injured/laminectomy site as a epicenter was then dissected out and post-fixed in 0.1 M PBS containing 4% paraformaldehyde for 24 hours at 4℃ and then embedded in paraffin. Paraffin embedded spinal cord samples were cut longitudinally with a microtome, at 4 µm thick sections through full dorso-ventral dimension of cord.
All the procedures were performed at room temperature unless otherwise specified. The cut free floating sections were mounted on poly-L-amino coated slides. After deparaffinization, sections were rehydrated in a microwave oven at 85℃ for 20 minutes, then xylene I, II, 100% ethanol I, II were used for 15 minutes each respectively to clean sections. 3% H2O2 was used for 10 minutes to quench endogenous peroxidase. Descending concentrations (95%, 90%, 80%, and 70%) of ethanol were used for 10 minutes respectively for dehydration. Sections were then rinsed two times in distilled water for 5 minutes each, 0.01 mol/L citrate buffer solution (pH 6.0) was added and microwaved in 'medium high' heat for 5 minutes and 'medium low' heat for 15 minutes, allowed to cool to room temperature and again rinsed 3 times in 0.1 M PBS for 5 minutes each. Incubation with 5% bovine serum albumin (BSA) blocking solution was done for 20 minutes to block non-specific binding. Sections were incubated overnight at 4℃ refrigerator after adding primary antibody mouse-anti-rat NF-κB P65 monoclonal antibody (1 : 100; Boster, Wuhan, China). Activated P65 in a NF-κB dimer is exclusively identified by this antibody. Sections were again rinsed 3 times in 0.1 M PBS for 5 minutes each, incubated with secondary antibody anti-mouse IgM (Boster, Wuhan, China) for 15-20 minutes, rinsed 3 times in 0.1 M PBS for 5 minutes each, strept avidin biotin complex added and incubated at 37℃ for 30 minutes, rinsed 4 times in 0.1 M PBS for 5 minutes each. Diaminobenzidine was applied for 3-5 minutes until brown colored reaction product was observed. After rinsing sections in distilled water 3 more times, they were counterstained with hematoxylin for 10 seconds, rinsed 3 times in distilled water, dehydrated with ascending concentration (i.e., 70%, 80%, 90%, 95%, and 100%) of ethanol for 10 minutes each, cleaned, mounted and then cover-slipped for immunohistochemical study. Analysis of equivalent regions for each section was done to analyze immunohistochemical marker qualitatively and quantitatively.
Each section was first examined under 100× magnifications to detect the highest positively expressed cells within the sections. Then 5 highly positive expressed visual fields were selected and observed under 400× magnifications. Analysis of the images was done using Image-Pro-Plus 6.0 software (Media Cybernetics, Bethesda, MD, USA). Total number of positive cells in each slide was calculated.
The level of NF-κB messenger RNA expression was determined by RT-PCR. Total RNA from rat's spinal cord was extracted using RNAiso plus (TaKaRa, Dalian, China) according to manufacturer's instructions. All the samples were treated with RNase free DNase-I (TaKaRa, Dalian, China) to confirm that all RNA samples were DNA free. RNA samples were quantified spectrophotometrically by measuring the absorbance at 260 nm and 280 nm. Following manufacturer's instruction, first strand of complentary DNA (cDNA) was synthesized by reverse transcribing 1 µL of total RNA into each 20 µL reaction volume using oligo DT-Adaptor primer and AMV Reverse Transcriptase (TaKaRa, Dalian, China). First strand cDNA (1 µL) was amplified in 10 µL PCR reaction volume containing 0.05 µL TaKaRa Ex Taq™ HS (Takara, Dalian, China), and 0.1 µL NF-κB primers, Forward 5' GGCAGCACTCCTTATCAA 3' Reverse 5' TGTCGTCCCAT CGTAGGT 3', GADPH primers, Forward 5' ACAGCAACAGG GTGGTGGAC 3' Reverse 5' TTTGAGGGTGCAGCGAACTT 3'. Samples were subjected to 104℃ for 5 minutes, 40 cycles each of 30 seconds at 94℃, 30 seconds at 54℃ and 30 seconds at 72℃ and 1 cycle of 10 minutes at 72℃ thermo cycler. PCR reactions lacking primers or reverse transcriptase were taken as negative controls. The specificity of the amplified RT-PCR product was confirmed on agarose gel electrophoresis. Each RT-PCR product was observed on 2% agarose gel containing 0.5 µg/mL ethidium bromide under ultraviolet light. Image J program (National Institute of Health, Bethesda, MD, USA) was used for quantification of band intensities.
Statistical analysis was performed using statistical program SPSS 20.0 software (SPSS, Chicago, IL, USA). All the data are presented as mean±SD values. For statistical analysis of results of behavioral analysis, immunohistochemical and RT-PCR analysis Kruskal-Wallis analysis of variance followed by Mann-Whitney U test for comparing multiple data from different groups were used. A p value <0.05 is considered to be significant statistically.
Diaminobenzidine immunohistochemistry was used for investigating cells positive for activated NF-κB in spinal cord at various time points (6 hours, 1 day, 3 days, 7 days, and 14 days) after SCI. Total NF-κB P65 positive cells were calculated. Few NF-κB P65 positive cells were detected in sham-surgery group at various time point after SCI. Significant increase in number of NF-κB P65 positive cells was exhibited in rats' spinal cord of experimental control group in comparison to that of sham surgery group (p<0.05), Significant reduction in the number of activated NF-κB P65 positively expressed cells were exhibited in rats' spinal cord of experimental group when compared to experimental control group (p<0.05). NF-κB P65 positive cells were detected in the control group at 6 hours and its expression was highest at 1 day after SCI and then kept on decreasing till 14 days after SCI (Fig. 1). At 6 hours after SCI, immunoreactivity of NF-κB was seen primarily within and adjacent to the transected margin, with almost negligible or little beyond this region. At 1 day and 3 days after SCI, immunoreactivity of NF-κB was seen through-out whole length of the spinal cord section (Fig. 2).
Confirmation of results of immunohistochemical examination and analysis of NF-κB expression at mRNA level at various time points after SCI in each group was done by RT-PCR. A basal level of NF-κB mRNA was detected in spinal cord from sham surgery group, where as NF-κB level was significantly increased in control group. BMMNCs transplanted model had less NF-κB mRNA expression in comparison to control group (p value<0.05) (Fig. 3). The amplified bands showed their predicted sizes : NF-κB 247 bp (Fig. 4A) and GADPH 252 bp (Fig. 4B).
All rats had normal limb function and obtained a BBB score of 21 before SCI. There was no locomotor dysfunction in rats from sham surgery group and obtained a score of 21 through-out the study. Serious hind limb locomotor dysfunction (complete paralysis) was detected in rats from experiment group and experiment control group in comparison to sham surgery group (p<0.05) at 1 day, 1 week, and 2 weeks post SCI. Rats from experimental group showed movement of 3 joints of hind limbs at 5th week after SCI (Fig. 5). This shows that BMMNCs treatment has a significant role in locomotor function recovery in comparison to vehicle treatment.
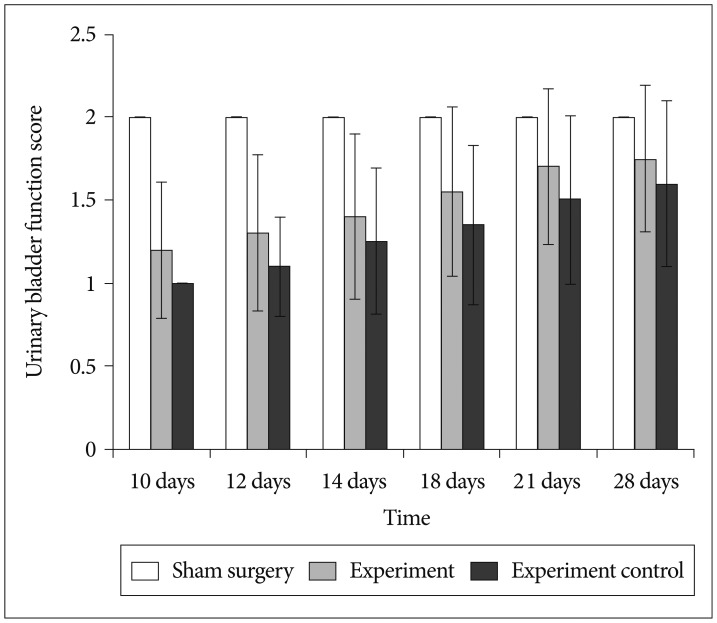
All the rats before SCI had normal bladder function. All rats from sham surgery group showed normal urinary bladder function through-out the study period. All rats from both group (experiment and experiment control) had severe urinary bladder dysfunction till 1 week after SCI. Earliest improvement in urinary bladder function was detected at 10th day after SCI in rats from experiment group. Rats from experiment group have significantly higher urinary bladder function in comparison to experiment control group (p<0.05). This signifies the role of BMMNCs in the recovery of urinary bladder function in comparison to vehicle treatment (Fig. 6).
Till date, traumatic SCI are managed with many challenges and with limited options. SCI leads to lifelong disability and no appropriate treatment exists for treating victims or minimizing their sufferings. The underlying mechanism leading to damage in SCI has not yet been fully understood. Inflammation and apoptosis evoked after SCI is known to significantly contribute to the fate of neurological outcome after SCI3). Inflammation and apoptosis both rely on their respective expression of genes. Inflammatory response induced after SCI leads to scar tissue formation and necrosis or apoptosis of neurons and oligodendrocytes, resulting in further waning of functional outcome7,15). The time period between SCI (first impact in trauma) and onset of secondary injury provides therapeutic time window of favorable circumstance for intervention. Thus role of various neuroprotective and neuro-regenerative agents have been studied in the neurological and functional outcome after SCI in animal models. In this study, we used BMMNCs as neuroprotective agent to provide a biological rationale for its' further studying in clinical trials. NF-κB has a pivotal role in regulating many genes liable for generating mediators and proteins responsible for secondary injury in SCI35).
Bethea et al.4) first demonstrated that the traumatic SCI induces NF-κB activation. He noted that NF-κB was activated within 30 minutes after injury and presented till 72 hours after injury. There are various possible subsequent targets of NF-κB which contributes in the modulation of apoptosis and response to stress28). Various inflammatory mediators such as cytokines, cellular adhesive molecules, inducible niric oxide synthetase (iNOS) and protease, which perpetuates and intensifies an inflammatory reaction, are up-regulated by NF-κB heterodimer P50/Rel A18). Several studies have reported that activated NF-κB was expressed in Central Nervous System by astrocytes, microglia, neurons and oligodendrocytes20,27). NF-κB transcription factor has been characterized as pluripotent factor in inflammatory response because various NF-κB related binding sequences have been detected on various genes with important immunological functions19). Recent researches also have demonstrated that SCI induces the activation of NF-κB in the spinal cord10,14,18,22).
Primary regulation of pro-inflammatory cytokines production is at transcriptional level. NF-κB family of transcription factors is the pivotal intermediate in transcriptional modulation of cytokine gene expression4). It has been now widely approved that pro-inflammatory cytokines formation, adhesion molecules expression on endothelium and neutrophils and vasoactive mediators (e.g., nitric oxide by iNOS, eicosanoids via COX-2) overproduction play crucial roles in inflammatory reactions after SCI19).
Till date various researches have demonstrated an interconnection between inhibition of activation of NF-κB and neuroprotection17,26,32). Inhibiting NF-κB activation pathway inhibits various inflammatory cytokines, chemokines, apoptotic factors, enzymes and mediators that are responsible for oxidative stress, inflammation, cytotoxicity, ultimate cell death, apoptosis of oligodendrocytes and neurons, glial scar formation, increased micro-vascular permeability and edema formation, neuropathic and inflammatory pain9,10,11,12,13,18,23,29,33). Loss of oligodendrocytes causes demyelination and this loss of myelin predisposes axons to noxious surroundings, resulting in neuronal loss due to necrosis/apoptosis. Degree of demyelination and axonal destruction is responsible for deterioration in locomotor function after traumatic SCI7,18).
Brambilla et al.5) in 2005 generated GFAP-IκB a-dn transgenic mice and SCI was established in those transgenic mice. Histologically significant white matter preservation and impressive functional improvement were seen in those transgenic mice in comparison to wild type mice. Rafati et al.29) in 2007 also concluded that SCI induces P65/P50 activity in injured spinal cord, which further stimulated the transcription of COX-2 and iNOS. P65/P50 targeted DNA decoy treatment attenuated COX-2 and iNOS protein level in injured cord and enhanced locomotor recovery.
Thus, these studies conclude that inhibition of NF-κB dependent cascades especially can limit progression of damage and enhances functional recovery in SCI by reducing inflammation, apoptosis, vascular permeability and chronic gliotic response, providing favorable environment, improving axonal sparing and sprouting and neuropathic pain6).
Vaquero et al. showed that bone marrow stromal cells injected directly into the site of contusion injury lead to superior functional recovery in the comparison to intravenous injection of BMSCs11). Here in contrast, we transplanted BMMNCs into normal parenchyma (-1 cm cranial and caudal) adjacent to lesion site to improve BMMNCs survival because injury epicenter may not provide favorable environment due to presence of phagocytic cell population. Rats in BMMNCs treatment group could move 2-3 joints of hind limbs at 5 weeks post SCI and showed earlier improvement in bladder function, whereas rats in vehicle treatment group could move only 1 joint of hind limbs and severe bladder dysfunction after SCI. An improvement in motor and urinary bladder function dictate the potential therapeutic effect of BM-MNCs after acute spinal cord injury. Spinal cord specimen of rats from sham surgery group had low NF-κB activity whereas it was considerably increased in injured cord from control group. In present study the time course of NF-κB activation is consistent with important role of NF-κB in triggering subsequent inflammatory cascade after SCI. In this study we showed that BMMNCs transplantation can reduce expression of activated NF-κB in the spinal cord after SCI, which is the first study to support that BM- MNCs can attenuate secondary injury in SCI and this explains the neuroprotective role of BMMNCs. At 6 hour post injury, NF-κB immunoreactivity was detected only within and adjacent to transected end but 72 hours post injury, NF-κB immunoreactivity was detected through-out the extent of spinal cord section (-20 mm), which is the hallmark of spinal cord injury. Maximum level of activated NF-κB immunoreactivity and NF-κB mRNA expression was detected at 1 day post injury. Spinal cord sample from BMMNCs treatment group showed decreased NF-κB immunoreactivity and mRNA expression in all time point when compared to control group.
Recent researches have reported the effective role of BMMNCs transplantation in improving neurological and functional outcome in animals and human patients after SCI37).
In conclusion, reduction in inflammation, attenuation of apoptosis of neurons, oligodendrocytes and inhibitory factors in regeneration and improvement in functional recovery can be achiev-ed by limiting NF-κB activation after SCI. We propose that im-provement in behavioral outcomes after SCI with BMMNCs is related to its role in suppressing the expression of activated NF-κB. But, the exact mechanism of action of BMMNCs is neither fully understood nor explained. So further researches to explain the mechanism is still warranted.
Acknowledgements
This work was supported by grant from Natural Science Foundation of Heilongjiang province (D201160) and Educational Foundation of Heilongjiang province, China (12511539).
References
1. Baeuerle PA. The inducible transcription activator NF-kappa B : regulation by distinct protein subunits. Biochim Biophys Acta. 1991; 1072:63–80. PMID: 2018779.

2. Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995; 12:1–21. PMID: 7783230.

3. Beattie MS. Inflammation and apoptosis : linked therapeutic targets in spinal cord injury. Trends Mol Med. 2004; 10:580–583. PMID: 15567326.

4. Bethea JR, Castro M, Keane RW, Lee TT, Dietrich WD, Yezierski RP. Traumatic spinal cord injury induces nuclear factor-kappaB activation. J Neurosci. 1998; 18:3251–3260. PMID: 9547234.
5. Brambilla R, Bracchi-Ricard V, Hu WH, Frydel B, Bramwell A, Karmally S, et al. Inhibition of astroglial nuclear factor kappaB reduces inflammation and improves functional recovery after spinal cord injury. J Exp Med. 2005; 202:145–156. PMID: 15998793.

6. Brambilla R, Hurtado A, Persaud T, Esham K, Pearse DD, Oudega M, et al. Transgenic inhibition of astroglial NF-kappa B leads to increased axonal sparing and sprouting following spinal cord injury. J Neurochem. 2009; 110:765–778. PMID: 19522780.

7. Cao HQ, Dong ED. An update on spinal cord injury research. Neurosci Bull. 2013; 29:94–102. PMID: 23124646.

8. Chen LF, Greene WC. Shaping the nuclear action of NF-kappaB. Nat Rev Mol Cell Biol. 2004; 5:392–401. PMID: 15122352.
9. Esposito E, Paterniti I, Mazzon E, Genovese T, Galuppo M, Meli R, et al. MK801 attenuates secondary injury in a mouse experimental compression model of spinal cord trauma. BMC Neurosci. 2011; 12:31. PMID: 21492450.

10. Genovese T, Paterniti I, Mazzon E, Esposito E, Di Paola R, Galuppo M, et al. Efficacy of treatment with verbascoside, biotechnologically produced by Syringa vulgaris plant cell cultures in an experimental mice model of spinal cord trauma. Naunyn Schmiedebergs Arch Pharmacol. 2010; 382:331–345. PMID: 20799028.

11. Glazova M, Pak ES, Moretto J, Hollis S, Brewer KL, Murashov AK. Pre-differentiated embryonic stem cells promote neuronal regeneration by cross-coupling of BDNF and IL-6 signaling pathways in the host tissue. J Neurotrauma. 2009; 26:1029–1042. PMID: 19138107.

12. Hamada Y, Ikata T, Katoh S, Tsuchiya K, Niwa M, Tsutsumishita Y, et al. Roles of nitric oxide in compression injury of rat spinal cord. Free Radic Biol Med. 1996; 20:1–9. PMID: 8903674.

13. Han X, Lu M, Wang S, Lv D, Liu H. Targeting IKK/NF-κB pathway reduces infiltration of inflammatory cells and apoptosis after spinal cord injury in rats. Neurosci Lett. 2012; 511:28–32. PMID: 22289688.

14. Han X, Wang S, Zhang Z, Lü D, Liu H. BMS-345541 inhibited nuclear factor kappa B expression and improved locomotor function recovery in rats after acute spinal cord injury. Neural Regen Res. 2011; 6:1775–1779.
15. Hausmann ON. Post-traumatic inflammation following spinal cord injury. Spinal Cord. 2003; 41:369–378. PMID: 12815368.

16. Jia Z, Zhu H, Li J, Wang X, Misra H, Li Y. Oxidative stress in spinal cord injury and antioxidant-based intervention. Spinal Cord. 2012; 50:264–274. PMID: 21987065.

17. Kerr BJ, Girolami EI, Ghasemlou N, Jeong SY, David S. The protective effects of 15-deoxy-delta-(12,14)-prostaglandin J2 in spinal cord injury. Glia. 2008; 56:436–448. PMID: 18205174.

18. Kim GM, Xu J, Xu J, Song SK, Yan P, Ku G, et al. Tumor necrosis factor receptor deletion reduces nuclear factor-kappaB activation, cellular inhibitor of apoptosis protein 2 expression, and functional recovery after traumatic spinal cord injury. J Neurosci. 2001; 21:6617–6625. PMID: 11517251.

19. La Rosa G, Cardali S, Genovese T, Conti A, Di Paola R, La Torre D, et al. Inhibition of the nuclear factor-kappaB activation with pyrrolidine dithiocarbamate attenuating inflammation and oxidative stress after experimental spinal cord trauma in rats. J Neurosurg Spine. 2004; 1:311–321. PMID: 15478370.

20. Lee KM, Jeon SM, Cho HJ. Tumor necrosis factor receptor 1 induces interleukin-6 upregulation through NF-kappaB in a rat neuropathic pain model. Eur J Pain. 2009; 13:794–806. PMID: 18938092.

21. Liebscher T, Schnell L, Schnell D, Scholl J, Schneider R, Gullo M, et al. Nogo-A antibody improves regeneration and locomotion of spinal cord-injured rats. Ann Neurol. 2005; 58:706–719. PMID: 16173073.

22. Mandalari G, Genovese T, Bisignano C, Mazzon E, Wickham MS, Di Paola R, et al. Neuroprotective effects of almond skins in experimental spinal cord injury. Clin Nutr. 2011; 30:221–233. PMID: 20864228.

23. Mao L, Wang H, Qiao L, Wang X. Disruption of Nrf2 enhances the upregulation of nuclear factor-kappaB activity, tumor necrosis factor-α, and matrix metalloproteinase-9 after spinal cord injury in mice. Mediators Inflamm. 2010; 2010:238321. PMID: 20862369.
24. Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999; 18:6853–6866. PMID: 10602461.

25. Parr AM, Tator CH, Keating A. Bone marrow-derived mesenchymal stromal cells for the repair of central nervous system injury. Bone Marrow Transplant. 2007; 40:609–619. PMID: 17603514.

26. Paterniti I, Genovese T, Crisafulli C, Mazzon E, Di Paola R, Galuppo M, et al. Treatment with green tea extract attenuates secondary inflammatory response in an experimental model of spinal cord trauma. Naunyn Schmiedebergs Arch Pharmacol. 2009; 380:179–192. PMID: 19337722.

27. Pizzi M, Spano P. Distinct roles of diverse nuclear factor-kappaB complexes in neuropathological mechanisms. Eur J Pharmacol. 2006; 545:22–28. PMID: 16854410.

28. Pollock G, Pennypacker KR, Mémet S, Israël A, Saporta S. Activation of NF-kappaB in the mouse spinal cord following sciatic nerve transection. Exp Brain Res. 2005; 165:470–477. PMID: 15912368.

29. Rafati DS, Geissler K, Johnson K, Unabia G, Hulsebosch C, Nesic-Taylor O, et al. Nuclear factor-kappaB decoy amelioration of spinal cord injury-induced inflammation and behavior outcomes. J Neurosci Res. 2008; 86:566–580. PMID: 17918744.

30. Schwaninger M, Inta I, Herrmann O. NF-kappaB signalling in cerebral ischaemia . Biochem Soc Trans. 2006; 34(Pt 6):1291–1294. PMID: 17073804.
31. Sekhon LH, Fehlings MG. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine (Phila Pa 1976). 2001; 26(24 Suppl):S2–S12. PMID: 11805601.

32. Sribnick EA, Wingrave JM, Matzelle DD, Wilford GG, Ray SK, Banik NL. Estrogen attenuated markers of inflammation and decreased lesion volume in acute spinal cord injury in rats. J Neurosci Res. 2005; 82:283–293. PMID: 16130149.

33. Tchivileva IE, Nackley AG, Qian L, Wentworth S, Conrad M, Diatchenko LB. Characterization of NF-kB-mediated inhibition of catechol-O-methyltransferase. Mol Pain. 2009; 5:13. PMID: 19291302.

34. The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. The People's Republic of China: The Ministry of Science and Technology of the People's Republic of China;2006.
35. Verma IM. Nuclear factor (NF)-kappaB proteins : therapeutic targets. Ann Rheum Dis. 2004; 63(Suppl 2):ii57–ii61. PMID: 15479873.
36. Willerth SM, Sakiyama-Elbert SE. Cell therapy for spinal cord regeneration. Adv Drug Deliv Rev. 2008; 60:263–276. PMID: 18029050.

37. Wright KT, El Masri W, Osman A, Chowdhury J, Johnson WE. Concise review : bone marrow for the treatment of spinal cord injury : mechanisms and clinical applications. Stem Cells. 2011; 29:169–178. PMID: 21732476.

Fig. 1
Total numbers of activated NF-κB positive cells in spinal cord of rats at different time post-injury. All bars represent mean values and error bars represent standard deviation. There were significantly increased activated NF-κB positive cells in experiment and experiment control group in comparison to sham surgery group (p<0.05) and significant decrease in experiment group in comparison to experimental control group (p<0.05). *Re-presents the time point of highest and significant difference in expression of NF-κB post-injury. NF-κB : nuclear factor-κB.

Fig. 2
A : Immunohistochemical analysis for the expression of NF-κB in experimental group at 6 hours post-injury. B : Immunohistochemical analysis for the expression of NF-κB in control group 6 hours post-injury. C : Immunohistochemical analysis for the expression of NF-κB in Experimental group at 1 day post-injury. D : Immunohistochemical analysis for the expression of NF-κB in control group at 1 day post-injury. E : Immunohistochemical analysis for the expression of NF-κB in Experiment group at 3 days post-injury. F : Immunohistochemical analysis for the expression of NF-κB in Control group at 3 days post-injury. G : Immunohistochemical analysis for the expression of NF-κB in experimental group at 7 days post-injury. H : Immunohistochemical analysis for the expression of NF-κB in control group at 7 days post-injury. I : Immunohistochemical analysis for the expression of NF-κB in experimental group at 14 days post-injury. J : Immunohistochemical analysis for the expression of NF-κB in control group at 14 days post-injury. K : Immunohistochemical analysis for the expression of NF-κB in Sham surgery group at 6 hours post-injury. L : Immunohistochemical analysis for the expression of NF-κB in Sham surgery group at14 days post injury. NF-κB : nuclear factor-κB.
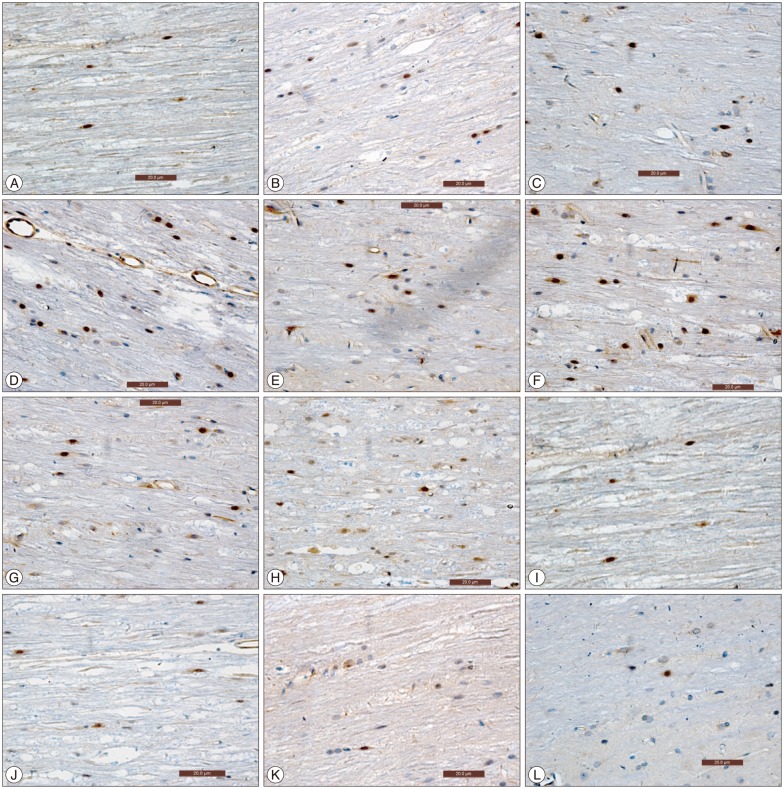
Fig. 3
Level of NF-κB mRNA expression in spinal cord of rats at different time post-injury. All bars represent mean values and error bars represent standard deviation. There were significantly increase in NF-κB mRNA level in experiment and experiment control group in comparison to sham surgery group (p<0.05) and significant decrease in experiment group in comparison to experimental control group (p<0.05). *Represents the time point of highest and significant difference in expression of NF-κB post-injury. NF-κB : nuclear factor-κB, mRNA : messenger RNA.

Fig. 4
A : Reverse transcription polymerase chain reaction (RT PCR) analysis of nuclear factor-κB expression in spinal cord (3 day post surgery). B : RT PCR analysis of GADPH expression in spinal cord (3 day post surgery). Lane 1 : marker, Lane 2 : Sham surgery group, Lane 3 : Experimental Control group, Lane 4 : Experimental group.
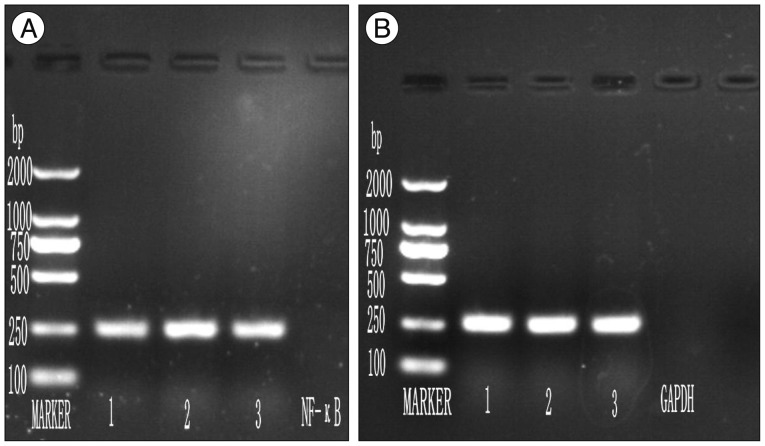
Fig. 5
Open field locomotor assessment by BBB score in rats at different time post-injury. All bars represent mean values and error bars represent standard deviation. There were significantly higher BBB score in rats from experiment group in comparison to experimental control group (p<0.05). BBB : Basso, Beattie, Bresnahan.
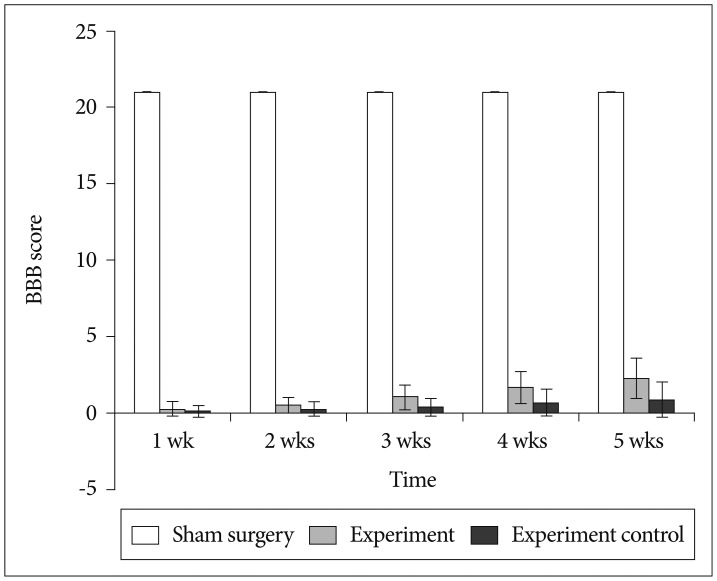
Fig. 6
Martin Schwab's Lab urinary bladder function score in rats at different time post-injury. All bars represent mean values and error bars represent standard deviation. There were significant urinary bladder function improvement in rats from experiment group in comparison to experimental control group (p<0.05).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download