Abstract
Objective
The purpose of this study was to describe the clinical characteristics, treatment outcomes, and prognostic factors in patients with brain abscesses treated in a single institute during a recent 10-year period.
Methods
Fifty-one patients with brain abscesses who underwent navigation-assisted abscess aspiration with antibiotic treatment were included in this study. Variable parameters were collected from the patients' medical records and radiological data. A comparison was made between patients with favorable [Glasgow Outcome Scale (GOS) ≥4] and unfavorable (GOS <4) outcomes at discharge. Additionally, we investigated the factors influencing the duration of antibiotic administration.
Results
The study included 41 male and 10 female patients with a mean age of 53 years. At admission, 42 patients (82%) showed either clear or mildly disturbed consciousness (GCS ≥13) and 24 patients (47%) had predisposing factors. The offending microorganisms were identified in 25 patients (49%), and Streptococcus species were the most commonly isolated bacteria (27%). The mean duration of antibiotic administration was 42 days. At discharge, 41 patients had a favorable outcome and 10 had an unfavorable outcome including 8 deaths. The decreased level of consciousness (GCS <13) on admission was likely associated with an unfavorable outcome (p=0.052), and initial hyperglycemia (≥140 mg/dL) was an independent risk factor for prolonged antibiotic therapy (p=0.032).
A brain abscess is a focal intracerebral infection that leads to a life-threatening condition with severe neurological deficits. Brain abscesses usually occur because of spreading from a contiguous focus of infection or hematogenous dissemination from a distant focus4,20,25). In general, the brain is resistant to bacterial and fungal infection because of an abundant blood supply and an impermeable blood-brain barrier. However, conditions that compromise the integrity of the central nervous system such as head trauma and neurosurgery can also cause brain abscesses8,28,30). In the past, brain abscesses were a uniformly fatal condition. However, the introduction of computed tomography (CT) in the early 1970s and the development of neurosurgical techniques along with the use of broad-spectrum antibiotics contributed to an improvement in patient prognoses6,24,26,34). The purpose of this study was to review the data of 51 patients with brain abscesses admitted to a single tertiary referral hospital during a recent 10-year period and describe their clinical characteristics, outcome of treatment, and prognostic factors.
This retrospective study included 51 patients with intracerebral abscesses treated with navigation-assisted abscess drainage at our institute between January 2004 and December 2013. A brain abscess was defined as the presence of one or more lesions with a hypodense center and peripheral ring enhancement, after the injection of contrast media, on CT or magnetic resonance images (MRIs). It was associated with at least one of the following characteristics: positive cultures of intracerebral materials, positive blood cultures, or histology of the intracerebral lesion suggesting a brain abscess. Patients with subdural empyema and epidural abscesses, and those who underwent craniotomy for brain abscesses, were excluded. Patient charts were reviewed for the following: age at diagnosis, sex, past history, symptom duration before diagnosis, level of consciousness [assessed by the Glasgow Coma Scale (GCS)], focal neurological status at admission, disease predisposition, various serum parameters [complete blood count, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and random blood sugar at admission], microorganisms identified on blood and abscess material cultures, antibiotic regimen, and duration of antibiotic treatment. The radiological parameters including the multiplicity of the lesion, abscess location, and initial and postoperative abscess volumes were also reviewed. Abscess volume was calculated using the abc/2 formula, where a is the greatest abscess diameter measured by CT or MRI, b is the diameter 90° to a, and c is the extent in the coronal direction or approximate number of slices within the abscess multiplied by the slice thickness (Fig. 1).
All patients underwent abscess aspiration under a neuronavigation system (StealthStation, Medtronic, Minneapolis, MN, USA). The operative intervention was performed at the earliest time, if indicated, without preoperative antibiotics so as not to sterilize the cultures. Patients who underwent delayed surgery were administered empirical antibiotics before the operation. During the operation, abscess material was maximally aspirated through a silicone catheter connected to soft collection bags placed in a dependent position and maintained for a minimum of 24 h. A repeat follow-up imaging study was performed routinely in all patients approximately 1-2 weeks postoperatively. Brain abscess specimens obtained from the surgery were cultured for aerobic and anaerobic bacteria, mycobacteria, and fungi. Antibiotic susceptibility tests were performed on the isolated microorganisms. Immediately after the abscess drainage procedure, the administration of empirical antibiotics was started intravenously until the pathogens were identified. The antibiotic regimen was then targeted against the specific organisms isolated in the culture. The choice of antibiotics for cases with sterile cultures was directed to the most likely organisms based on clinical history and the primary site of infection, if known. The referring physician was later consulted with regard to adjustments or additions to the antibiotics and maintenance of the agents. The outcome was assessed according to the Glasgow Outcome Scale (GOS) at the time of discharge. A favorable outcome (GOS ≥4) was defined as moderate disability (having a disability, but being independent) and good recovery. An unfavorable outcome (GOS <4) was defined as death, persistent vegetative status, or severe disability (conscious, but with disability).
Statistical analysis was performed using the Statistical Package for the Social Sciences software (SPSS 11.5, IBM Corporation, Armonk, NY, USA). Results are presented as the mean±standard deviation of the mean. Univariate analyses (chi-square test, Fisher's exact test, Student's t-test) were performed to compare the variables between patients with a favorable outcome or with early response to antibiotics, and those with poor outcomes or with a delayed response to antibiotics. Variables found to have p<0.1 in the univariate analyses were entered into a multivariate logistic regression model to identify independent predictors of neurological outcome and the duration of antibiotic use. The correlation between continuous variables was assessed using Pearson's correlation coefficient. Data were considered statistically significant when their p-value was below 0.05.
The patient demographics are listed in Table 1. There were 41 male and 10 female patients with a mean age of 53 years (range: 14-81 years). Headache, which occurred in 25 patients, was the most common symptom, while 3 patients complained of nausea and vomiting. Motor weakness occurred in 20 patients, and fever was checked in 10 patients on admission. Other symptoms and signs included speech disturbance (8 patients), visual disturbance (4 patients), impaired cognition (3 patients), seizures (2 patients), malaise (2 patients), and sensory changes (2 patients). Clear or mildly disturbed consciousness (GCS ≥13) was evident in 42 patients (82%) and moderately or severely disturbed consciousness (GCS <13) was present in 9 patients (18%). The mean interval between symptom onset and admission was 8 days (range: 0-45 days). A total of 24 patients had predisposing factors including pulmonary infection (7 patients), sinusitis (4 patients), dental infection (3 patients), prior trauma or neurosurgery (2 patients), otic infection (2 patients), endocarditis (2 patients), congenital heart disease (2 patients), skin infection (1 patient), and psoas abscess (1 patient). Ten patients presented with known diabetes mellitus and 4 patients were in an immunocompromised state resulting from chemotherapy for cancer (2 patients), chronic renal failure (1 patient), and steroid therapy for Addison's disease (1 patient).
Of the 51 patients, leukocytosis [white blood cell (WBC) ≥10000/µL] at admission was identified in 24 (47%) patients. The mean WBC count was 11402±632/µL. Mean serum ESR and CRP levels at admission were 34.25±4.08 mm/h and 39.12±8.13 mg/L, respectively. An elevated CRP level (≥10 mg/L) was detected in 31 (61%) patients. The mean plasma glucose level at admission was 147.60±58.37 mg/dL. Twenty-two (43%) of the 51 patients were in a state of hyperglycemia (blood glucose ≥140 mg/dL) on admission.
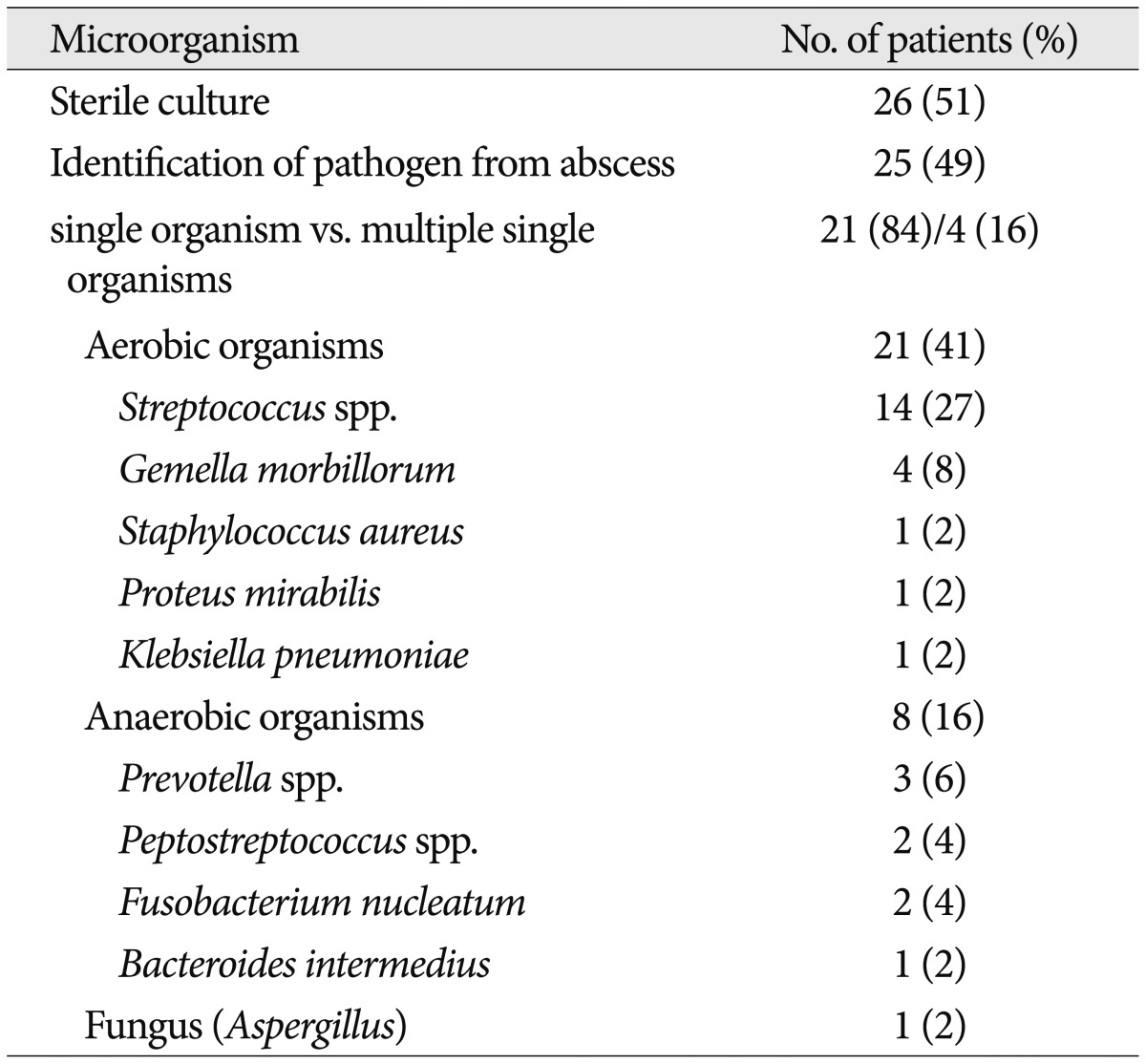
On brain images, a single lesion was observed in 44 (86%) of the 51 patients and multiple lesions were observed in 7 patients (14%). The abscesses were most commonly found in the frontal lobe (30 patients, 59%), followed by the parietal lobe (12 patients, 24%), temporal lobe (8 patients, 16%), occipital lobe (5 patients, 10%), thalamus (2 patients, 4%), and pons (1 patient, 2%). The mean preoperative abscess volume was 19.11±2.00 cm3 and the residual volume of the abscesses after surgery was 7.03±0.97 cm3 (Table 2).
Abscess pathogens were identified in 25 patients (49%) during a culture study of the abscess material (Table 3). A single organism was isolated from 21 patients and multiple organisms were isolated from 4 patients. Of these isolated microorganisms, 21 (41%) were aerobic and 8 (16%) were anaerobic bacteria. A fungus (Aspergillus) was isolated from 1 patient. The most common aerobic bacteria were the Streptococcus species (14 cases, 27%), followed by Gemella morbillorum (4 cases, 8%), Staphylococcus aureus (1 case, 2%), Proteus mirabilis (1 case, 2%), and Klebsiella pneumoniae (1 case, 2%). The most frequently isolated anaerobic bacteria belonged to the Prevotella species (3 cases, 6%), followed by the Peptostreptococcus species (2 cases, 4%), Fusobacterium nucleatum (2 cases, 4%), and Bacteroides intermedius (1 case, 2%). Blood cultures were performed in 10 patients who presented with fever at admission and were positive in 2 patients (4%). In one patient, Staphylococcus aureus was isolated from the blood, as well as from the abscess material. In the other patient, the abscess fluid culture was negative, and the blood culture showed Streptococcus viridans growth. Once this pathogen was identified, the patient's antibiotic therapy was revised accordingly.
The mean duration between admission and surgery was 7.29 days (range: 1-27 days). Of the 51 patients, 6 patients (11.8%) underwent surgery twice because a considerable portion of the abscess remained after the first intervention. Intravenous administration of empirical antibiotics including vancomycin, ceftriaxone, and metronidazole were started after abscess aspiration until the pathogens were confirmed. In the 8 patients who were suspected to be in the cerebritis stage, the antibiotics were started before surgery. The most commonly used antibiotics included a combination of ceftriaxone and metronidazole (22 patients, 43%), sometimes with the addition of either vancomycin or gentamicin. The mean duration of intravenous antibiotic treatment for surviving patients was 42 days (range: 23-66 days). None of the patients received oral antibiotics after they completed a course of parenteral antibiotic therapy. Corticosteroids were selectively administered to patients with severely increased intracranial pressure (9 patients, 18%). During the course of treatment, 8 patients (16%) died, with 3 (6%) deaths being attributed to the abscess itself and 5 (10%) deaths that may have resulted from the underlying pathology. In the latter cases, the cause of death was attributable to the aggravation of a pre-existing disease: 2 patients died of pulmonary infection-related sepsis, 1 patient with an atrial septum defect died from a pulmonary embolism, 1 patient with congestive heart failure died from a sudden heart attack, and 1 patient who had hepatoma died from bleeding of the original tumor. At discharge, a favorable outcome (GOS≥4) was observed in 41 patients. Of the survivors, 2 patients had unfavorable outcomes (GOS<4). One of the patients with a thalamic abscess had a severe neurological deficit and was unable to live independently, and the other remained in a persistent vegetative state due to secondary ventriculitis caused by a rupture of the abscess.
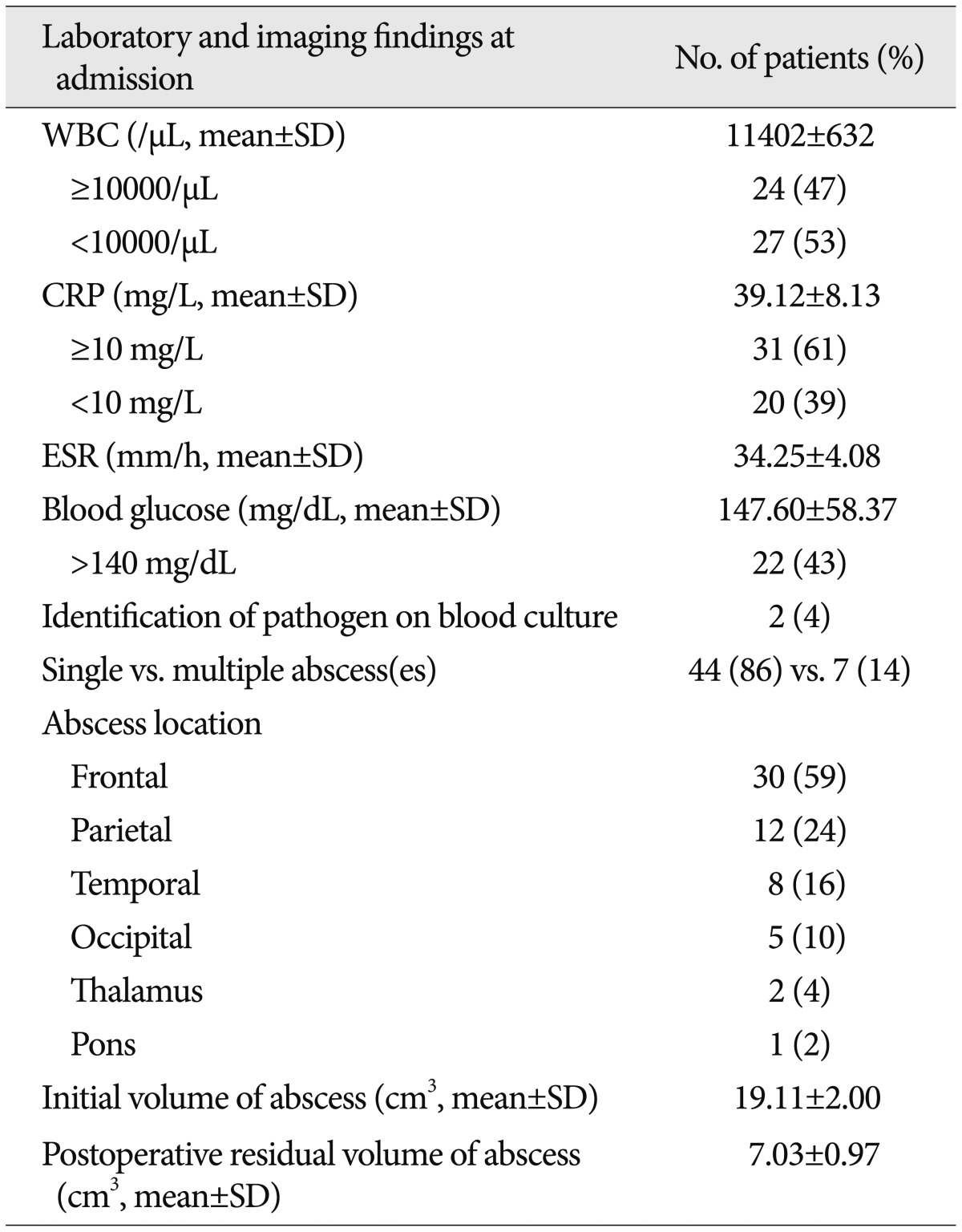
The results of comparison analyses between the patients with favorable and unfavorable outcomes are shown in Table 4. In the univariate analyses, admission variables associated with patient outcome included an initial GCS score <13 (p=0.003), a high level of serum CRP (≥10 mg/L, p=0.024), and hyperglycemia (≥140 mg/dL, p=0.016). Among these, only a GCS score <13 [p=0.052, 95% confidence interval (CI) 0.982-37.118] was likely to be an independent risk factor for poor patient outcome in the multivariate logistic regression analysis.
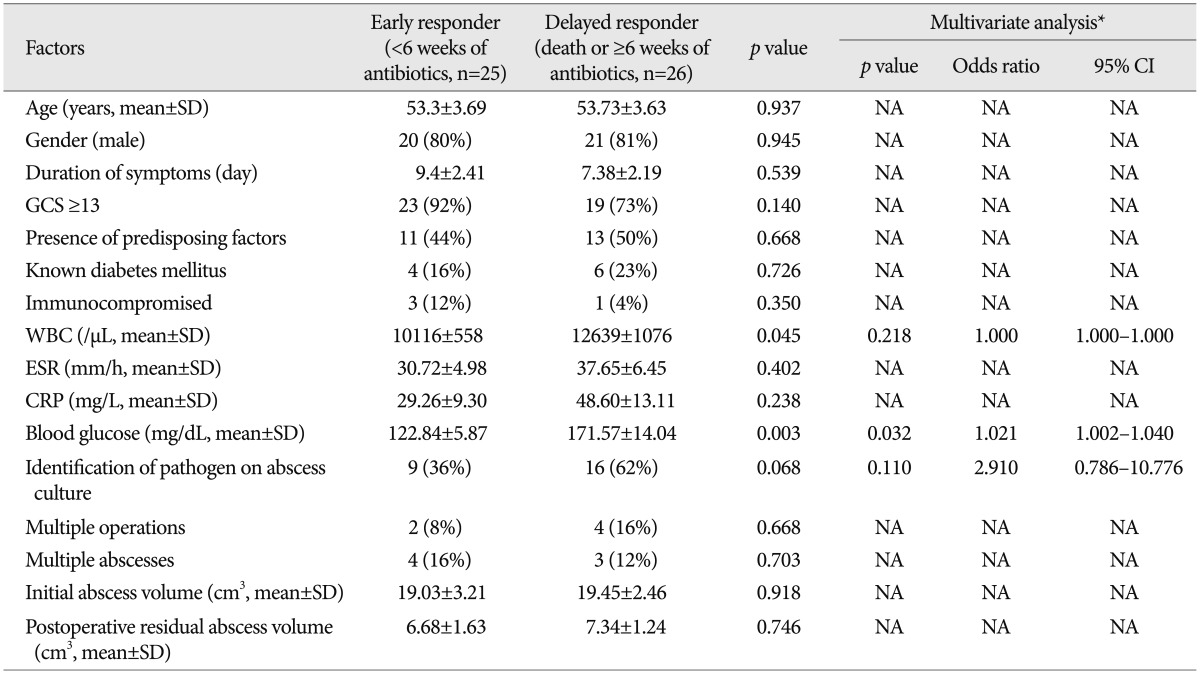
To assess the clinical and radiological factors that were associated with the response to antibiotics, the patients were divided into two groups: 1) early responder group (duration of antibiotics <6 weeks) comprising 25 patients, and 2) delayed responder group (dead or using antibiotics for ≥6 weeks) comprising 26 patients. The mean WBC count and plasma blood sugar level in the delayed responder group were significantly higher than in the early responder group (12639/µL vs. 10116/µL, p=0.045; 171.57 mg/dL vs. 122.84 mg/dL, p=0.003, respectively) (Table 5). In the multivariate analyses, the independent risk factor for prolonged antibiotic use was hyperglycemia (p=0.032, 95% CI 1.002-1.040). There was a positive correlation between the initial blood sugar level and the duration of antibiotic use (r=0.471 and p=0.001) (Fig. 2).
Brain abscesses are considered serious and life threatening infections. The mortality rate associated with brain abscesses was reported to be as high as 40% in the 1980s8). Advances in radiographic scanning, the development of novel surgical techniques, and the availability of newer antibiotics have all helped decrease the mortality rate in patients with brain abscesses6,24,26,34). Recently, Tseng and Tseng31) have published the clinical outcomes of their patients with brain abscesses (n=142), of which 16.9% died during hospitalization. The deaths were caused by underlying systemic infection or the terminal stage of cancer and not by the abscess itself. Similarly, in our series, the overall mortality rate was 16% (8 patients) and the majority of these deaths were related to systemic infections, heart attacks, or aggravation of their pre-existing diseases. Only 3 patients died because of the brain abscess itself. This finding suggests that, although the current therapeutic strategy for controlling these abscesses is highly effective, the decisive factor for patients' survival still depends on their ability to recover from their medical comorbidities.
Headache has been reported as the most common presenting symptom of brain abscesses20). Several case series have reported headache, fever, and altered mental status as the most frequent presenting symptoms, while less commonly observed symptoms include nausea, vomiting, and focal neurological symptoms and signs15,18,25). However, Kao et al.15) reported that fever was not helpful diagnostically. In a study for patients with pneumococcal brain abscesses, fever was found in only 29% of patients, while 86% had focal deficits and 81% had headaches12). In the present series, headache was the most frequent single symptom (49%). Forty percent of patients had motor weakness, either hemiparesis or monoparesis, 18% had moderately or severely disturbed consciousness, and 16% had speech disturbances. Fever was present in 20% of the patients. Overall, it seems that headache and focal neurological deficits including motor deficits and dysarthria are the most frequent presenting symptoms, with fever being common, but not invariable, since the systemic inflammatory response to an abscess may be minimal. Chun et al.8) demonstrated that old age, male gender, altered sensorium on admission, and abscesses with a pulmonary source were associated with poor outcomes in patients with brain abscesses. Other studies found that the mortality among treated patients correlated significantly with the initial neurological grade20,34). Seydoux and Francioli30) also revealed that death and severe sequelae were related to the severity of neurological impairment on admission and to a shorter duration from symptom onset to admission. In the present study, patients with a low GCS score on admission had a greater likelihood of an unfavorable outcome than those with minimal mental disturbances when all potential confounds had been adjusted for in the multivariate analysis.
Cerebral abscesses can occur in a variety of clinical settings. Direct spreading from an ear infection, sinusitis, or dental infection is the major cause for developing an abscess. This contiguous spreading of infection from adjacent sites has been reported in nearly half of all brain abscess cases in the past2,8,14). However, in our series, only 13 (26%) of the patients had a contiguous focus of infection as their predisposing cause (4 sinus, 3 dental, and 2 ear infections). Similar to our findings, Carpenter et al.6) also found that 24% of patients with brain abscesses had primary infections in their head and neck. Aggressive and widespread therapy for the primary infections in the adjacent area is likely to lead to a corresponding decrease in the incidence of brain abscesses associated with these conditions. In addition to a documented focus of infection, patients with certain medical conditions have an increased risk of infection15,18). Many of these conditions, including diabetes and cancer, reduce the immune response in the patient. In this series, 14 (28%) patients had a medical condition that might have increased their susceptibility to infection: diabetes mellitus in 10 patients and an immunocompromised state in 4. This is in accord with other series, in which diabetes has been shown to be an important predisposing factor15,18). Kao et al.15) reported that patients with underlying diabetes and liver disease had a higher mortality. However, in the present study, we failed to find any apparent correlation between outcomes and the presence or type of predisposing factors.
Identifying the offending pathogens is crucial for managing abscesses more efficiently. However, a lumbar puncture to obtain cerebrospinal fluid may be contraindicated in a patient with a brain abscess because raised intracranial pressure may lead to brainstem herniation and death20,30). Blood cultures are often helpful in some patients, especially those who did not undergo an operation, and in patients whose abscess fluid cultures were negative. In addition, if the abscess is thought to be the result of hematogenous spreading, a blood culture is indicated.
The introduction, in the 1980s, and subsequent widespread acceptance of CT-guided stereotactic aspiration for brain abscesses, a minimally invasive procedure with low morbidity and mortality, has allowed rapid and effective surgical drainage of many brain abscess cases. In turn, this has enabled the culturing of pus and the identification of the organism(s) responsible, with high rates of resolution and a good outcome. Some studies have advocated nonsurgical treatments for patients who are poor candidates for surgery or for those with surgically inaccessible lesions3). The major drawback to this approach is the potential toxicity involved in the prolonged administration of empirical antimicrobial therapy20). Even in regions with limited accessibility such as the brain stem or eloquent areas among patients with a high risk for surgery or with multiple brain abscesses, stereotactic aspiration combined with antibiotic treatment can be safely applied in most cases9). In our center, we attempt to treat most of the lesions suspected to be brain abscesses on imaging and clinical manifestations by aspiration and drainage guided by navigation, except during the stage of cerebritis. This approach not only achieves a reduction of the mass effect, but also secures abscess materials for identifying the infecting pathogens, and thus for facilitating antibiotic selection16). Although the excision of abscesses may shorten the clinical course19), no further improvement in the outcome has been observed16,34).
There has been no laboratory data of any value for outcome prediction in patients with brain abscesses. Hyperglycemia is reportedly associated with a poor outcome in several systemic infectious and neurological disorders, such as sepsis, stroke, and severe head injury5,13,17,27). Intensive insulin therapy has been shown to prevent morbidity and reduce mortality in critically ill patients32,33). Elevated glucose in the blood has been associated with an increase in circulating cytokine concentrations and with an impaired ability to oppose infection11). Therefore, high blood glucose levels are regarded as an epiphenomenon of systemic disease severity and physical stress. On the contrary, there is evidence that hyperglycemia associated with brain pathology is a central phenomenon10). The hypothalamus is the most studied central site with respect to glucose metabolism1). Animal studies have shown that stimulation of the hypothalamus results in hyperglycemia10). Implicated in the pathogenesis is an increase in sympathetic nervous system activity and a subsequent change in glucose metabolism through the hypothalamic-pituitary-adrenal axis, which is integral to the neuroendocrine stress response1). A specific anatomical site responsible for governing glucose homeostasis has been presumed to exist21,22). Allport et al.1) reported that insular cortical strokes induce hyperglycemia. They suggested that the insular cortex influences autonomic function, especially the tone of the sympathetic nervous system, via its interconnections with the hypothalamus1,7,23). In a recent study, Schut et al.29) demonstrated that 69% of patients with bacterial meningitis have hyperglycemic blood glucose levels on admission. In addition, they showed an association between hyperglycemia on admission and patient outcomes. In the present study, we found that 43% of patients with brain abscess had an elevated glucose level (≥140 mg/dL) at the initial presentation. Although the exact mechanism remains uncertain, it can be assumed that central nervous system infection, including bacterial meningitis and brain abscesses may involve cortical and/or subcortical autonomic centers and affect the regulation of the autonomic nervous system, leading to hyperglycemia. Our study showed that hyperglycemia is related to an unfavorable outcome in the univariate analysis (p=0.016), but this relationship do not remain robust in a multivariate analysis (p=0.081). Interestingly, the patients who received prolonged antibiotic treatment (≥6 weeks) had a significantly higher glucose level at admission compared to those who had a relatively short period of antibiotic therapy (<6 weeks, 171.57 mg/dL vs. 122.84 mg/dL, p=0.003). In the multivariate analysis, elevated blood sugar on admission was an independent risk factor associated with prolonged antibiotic use (p=0.032, 95% CI=1.002-1.040). Finally, we found that the duration of antibiotic administration was directly correlated with the initial glucose level (r=0.471 and p=0.001). Other laboratory parameters regarded as indicators for the severity of systemic infection, such as WBC count, ESR, and CRP, were of limited value for predicting the patients' outcomes or the duration of antibiotic use in the statistical analysis. These findings suggest that hyperglycemia is likely the result of a central nervous system insult leading to disturbed blood-glucose regulation mechanisms, rather than the result of systemic insults; thus, hyperglycemia may be a useful predictor of treatment outcome and the responsiveness of postoperative medical treatment in patients with brain abscesses.
This study had several limitations inherent to its retrospective design. As with any observational study, there remains a possibility that unmeasured confounds influenced our findings. In addition, our study did not include patients who underwent a craniotomy with their abscess excision, which might have an independent effect on the outcome. We assessed a single random blood glucose level on admission to define hyperglycemia without measuring glycosylated hemoglobin (HbA1C). Although we attempted to define the medical condition of the patients clearly, including pre-existing diabetes, by performing a critical review of the patients' medical histories, it is likely that we also included patients with undiagnosed diabetes, which might have introduced an important bias in our analysis.
In this retrospective study of 51 patients with brain abscesses, we found that the GCS score on admission was associated with the patients' outcomes. The overall mortality rate was 16%; however, the majority of the deaths were caused by patients' pre-existing critical medical conditions and not by the abscesses themselves. In survivors, the response to a combination therapy of abscess drainage followed by parenteral antibiotic administration was excellent. An elevated blood glucose level on admission was associated with a prolonged use of antibiotics.
References
1. Allport LE, Butcher KS, Baird TA, MacGregor L, Desmond PM, Tress BM, et al. Insular cortical ischemia is independently associated with acute stress hyperglycemia. Stroke. 2004; 35:1886–1891. PMID: 15192241.

2. Arseni C, Ciurea AV. Cerebral abscesses secondary to otorhinolaryngological infections. A study of 386 cases. Zentralbl Neurochir. 1988; 49:22–36. PMID: 3043985.
3. Barsoum AH, Lewis HC, Cannillo KL. Nonoperative treatment of multiple brain abscesses. Surg Neurol. 1981; 16:283–287. PMID: 7302827.

5. Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. 2001; 32:2426–2432. PMID: 11588337.

6. Carpenter J, Stapleton S, Holliman R. Retrospective analysis of 49 cases of brain abscess and review of the literature. Eur J Clin Microbiol Infect Dis. 2007; 26:1–11. PMID: 17180609.

7. Cechetto DF. Identification of a cortical site for stress-induced cardiovascular dysfunction. Integr Physiol Behav Sci. 1994; 29:362–373. PMID: 7696133.

8. Chun CH, Johnson JD, Hofstetter M, Raff MJ. Brain abscess. A study of 45 consecutive cases. Medicine (Baltimore). 1986; 65:415–431. PMID: 3784900.
9. Dyste GN, Hitchon PW, Menezes AH, VanGilder JC, Greene GM. Stereotaxic surgery in the treatment of multiple brain abscesses. J Neurosurg. 1988; 69:188–194. PMID: 3292717.

10. Ellenberg M, Rifkin H. Diabetes Mellitus. Theory and Practice. ed 3. New Hyde Park, NY: Medical Examination Pub. Co.;1983. p. 267–294.
11. Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002; 106:2067–2072. PMID: 12379575.

12. Grigoriadis E, Gold WL. Pyogenic brain abscess caused by Streptococcus pneumoniae: case report and review. Clin Infect Dis. 1997; 25:1108–1112. PMID: 9402366.

13. Jeremitsky E, Omert LA, Dunham CM, Wilberger J, Rodriguez A. The impact of hyperglycemia on patients with severe brain injury. J Trauma. 2005; 58:47–50. PMID: 15674149.

14. Kangsanarak J, Navacharoen N, Fooanant S, Ruckphaopunt K. Intracranial complications of suppurative otitis media: 13 years' experience. Am J Otol. 1995; 16:104–109. PMID: 8579165.
15. Kao PT, Tseng HK, Liu CP, Su SC, Lee CM. Brain abscess: clinical analysis of 53 cases. J Microbiol Immunol Infect. 2003; 36:129–136. PMID: 12886965.
16. Kratimenos G, Crockard HA. Multiple brain abscess: a review of fourteen cases. Br J Neurosurg. 1991; 5:153–161. PMID: 1863376.

17. Kruyt ND, Roos YW, Dorhout Mees SM, van den Bergh WM, Algra A, Rinkel GJ, et al. High mean fasting glucose levels independently predict poor outcome and delayed cerebral ischaemia after aneurysmal subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 2008; 79:1382–1385. PMID: 18403438.

18. Lu CH, Chang WN, Lin YC, Tsai NW, Liliang PC, Su TM, et al. Bacterial brain abscess: microbiological features, epidemiological trends and therapeutic outcomes. QJM. 2002; 95:501–509. PMID: 12145389.

19. Mampalam TJ, Rosenblum ML. Trends in the management of bacterial brain abscesses: a review of 102 cases over 17 years. Neurosurgery. 1988; 23:451–458. PMID: 3200375.

20. Mathisen GE, Johnson JP. Brain abscess. Clin Infect Dis. 1997; 25:763–779. quiz 780-781. PMID: 9356788.

21. Mitchell AJ. Clinical implications of poststroke hypothalamo-pituitary adrenal axis dysfunction: a critical literature review. J Stroke Cerebrovasc Dis. 1997; 6:377–388. PMID: 17895038.

22. Murros K, Fogelholm R, Kettunen S, Vuorela AL. Serum cortisol and outcome of ischemic brain infarction. J Neurol Sci. 1993; 116:12–17. PMID: 8509800.

23. Oppenheimer S. The anatomy and physiology of cortical mechanisms of cardiac control. Stroke. 1993; 24(12 Suppl):I3–I5. PMID: 8249017.
24. Park SH, Lee SW, Kang DH, Hwang JH, Sung JK, Hwang SK. The role of f-fluorodeoxyglucose positron emission tomography in the treatment of brain abscess. J Korean Neurosurg Soc. 2011; 49:278–283. PMID: 21716900.

25. Roche M, Humphreys H, Smyth E, Phillips J, Cunney R, McNamara E, et al. A twelve-year review of central nervous system bacterial abscesses; presentation and aetiology. Clin Microbiol Infect. 2003; 9:803–809. PMID: 14616700.

26. Rosenblum ML, Hoff JT, Norman D, Weinstein PR, Pitts L. Decreased mortality from brain abscesses since advent of computerized tomography. J Neurosurg. 1978; 49:658–668. PMID: 712388.

27. Rovlias A, Kotsou S. The influence of hyperglycemia on neurological outcome in patients with severe head injury. Neurosurgery. 2000; 46:335–342. discussion 342-343. PMID: 10690722.

28. Schliamser SE, Bäckman K, Norrby SR. Intracranial abscesses in adults: an analysis of 54 consecutive cases. Scand J Infect Dis. 1988; 20:1–9. PMID: 3363298.

29. Schut ES, Westendorp WF, de Gans J, Kruyt ND, Spanjaard L, Reitsma JB, et al. Hyperglycemia in bacterial meningitis: a prospective cohort study. BMC Infect Dis. 2009; 9:57. PMID: 19426501.

30. Seydoux C, Francioli P. Bacterial brain abscesses: factors influencing mortality and sequelae. Clin Infect Dis. 1992; 15:394–401. PMID: 1520783.

31. Tseng JH, Tseng MY. Brain abscess in 142 patients: factors influencing outcome and mortality. Surg Neurol. 2006; 65:557–562. discussion 562. PMID: 16720170.

32. van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006; 354:449–461. PMID: 16452557.

33. van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001; 345:1359–1367. PMID: 11794168.

Fig. 1
A: Magnetic resonance image (MRI) of a patient with a 5.9×4.8×5.5 cm brain abscess in the left frontal lobe. The maximum perpendicular diameters (a and b) of the abscess were measured on axial MRIs and the extent in the coronal direction (c) was assessed on coronal or sagittal images. The volume of the abscess was then calculated using the formula: V=abc/2. B: The patient was managed with abscess drainage followed by parenteral antibiotic administration for 6 weeks. MRI at discharge showed a marked decrease in abscess volume and a restoration of mass effect by the abscess.
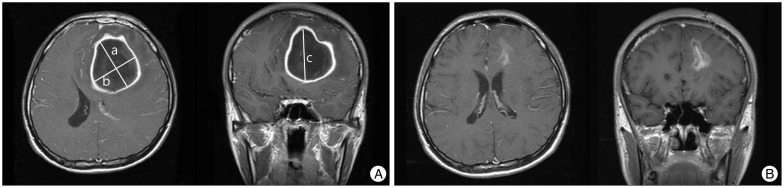
Fig. 2
Correlation between the blood glucose level at admission and the duration of antibiotic administration. A significant positive correlation was found between these two factors (r=0.471, p=0.001).

Table 4
Results of univariate and multivariate analyses for treatment outcomes in patients with brain abscesses




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download