Abstract
Objective
Postoperative delirium is a common complication in the elderly after surgery but few papers have reported after spinal surgery. We analyzed various risk factors for postoperative delirium after spine surgery.
Methods
Between May 2012 and September 2013, 70 patients over 60 years of age were examined. The patients were divided into two groups : Group A with delirium and Group B without delirium. Cognitive function was examined with the Mini-Mental State Examination-Korea (MMSE-K), Clinical Dementia Rating (CDR) and Global Deterioration Scale (GDS). Information was also obtained on the patients' education level, underlying diseases, duration of hospital stay and laboratory findings. Intraoperative assessment included Bispectral index (BIS), type of surgery or anesthesia, blood pressure, fluid balance, estimated blood loss and duration of surgery.
Results
Postoperative delirium developed in 17 patients. The preoperative scores for the MMSE, CDR, and GDS in Group A were 19.1±5.4, 0.9±0.6, and 3.3±1.1. These were significantly lower than those of Group B (25.6±3.4, 0.5±0.2, and 2.1±0.7) (p<0.05). BIS was lower in Group A (30.2±6.8 compared to 35.4±5.6 in group B) (p<0.05). The number of BIS <40 were 5.1±3.1 times in Group A, 2.5±2.2 times in Group B (p<0.01). In addition, longer operation time and longer hospital stay were risk factors.
Conclusion
Precise analysis of risk factors for postoperative delirium seems to be more important in spinal surgery because the surgery is not usually expected to have an effect on brain function. Although no risk factors specific to spinal surgery were identified, the BIS may represent a valuable new intraoperative predictor of the risk of delirium.
Among many types of delirium, postoperative delirium is a common and well-known complication of elderly patients after general anesthesia. Postoperative delirium can be related to adverse results, including functional dysfunction, increased health care costs, and, sometimes, postoperative mortality29).
According to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), delirium is defined as a "disturbance in attention and orientation to the environment that develops in a short period without other neurocognitive disorder" and as a "change in an additional cognitive domain"1). Although there are variations in the duration and the degree of delirium, postoperative delirium usually develops between 24 to 72 hours, and it can persist for several hours or days. Postoperative delirium should be differentiated from emergence delirium, which can be seen transiently during or immediately after recovery from general anesthesia31).
Postoperative delirium studies have usually been handled in anesthesiology, cardiac and orthopedic surgery fields. Only a few studies have focused on the spinal area or risk factors for delirium following spinal surgery. Therefore, compared with the occurrence of delirium with other types of postoperative surgeries, the risk factors for postoperative delirium following spinal surgery have not been fully clarified5,15,16,20,24,25,33). This circumstance, regarding the increasing cases of postspinal surgery delirium in elderly patients, makes it difficult to respond or explain the postoperative delirium and to prevent it perioperatively. Thus, in the present study, we examined diverse risk factors that affect postoperative delirium in elderly spinal-surgery patients.
Patients older than 60 years who underwent spinal surgery for degenerative spinal conditions between May 2012 and September 2013 were enrolled in this study. We selected elderly patients, because age is already known to be the most important risk factor for postoperative delirium10,18,21,24,33). We excluded patients with spinal tumor-like lesions, infection, or trauma that could affect the patients' general condition. Patients with known brain problems, including cerebral contusions, cerebrovascular diseases, Alzheimer's disease, and Parkinson's disease, all of which can cause disorientation, were also excluded. None of the patients had psychological problems. Among a total of 591 patients who underwent spinal surgeries, we selected and studied 70 patients retrospectively who met the inclusion criteria. Postoperative delirium was diagnosed according to DSM-5 criteria1). The patients were divided into two groups : Group A with postoperative delirium and Group B without postoperative delirium. Surgical procedures included fusion operation, decompressive laminectomy and discectomy of the lumbar spine, anterior cervical discectomy and fusion, laminoplasty and foraminotomy of the cervical spine. Patient-controlled analgesia (PCA) including fentanyl was used for all patients unless the patient refused the use of PCA. PCA was used in all patients except two patients, one patient from each group.
Cognitive function was assessed with the Mini-Mental State Examination-Korea (MMSE-K), the Clinical Dementia Rating (CDR), and the Global Deterioration Scale (GDS) one day before the surgery by a trained interviewer. The MMSE-K is a modification of the MMSE with scores ranging from 0 to 30, and a score of 23 or lower means cognitive impairment. The MMSE-K tests five cognitive functional areas : orientation, registration, attention-calculation, recall, and language14). The CDR evaluates six areas : memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care22). The CDR is a numeric scale system rating of 0, 0.5, 1, 2, 3, 4, and 5, and a score of 1 or higher represents cognitive impairment. The GDS identifies seven clinically recognizable stages of cognitive function from normal to severe cognitive dysfunction based mainly on thinking and functioning abilities26). The GDS is numbered according to stages 1 to 7, and the stage 3 or higher represents cognitive impairment.
We investigated the patient's history of hypertension or diabetes mellitus, educational level, and smoking. Hemoglobin, hematocrit, and electrolyte levels were checked preoperatively. The patient's education level was assigned a score of 0 for the non-educated and the others were calculated by the period of education by yearly basis. The preoperative duration of the hospital stay was checked as a number of admission days before the date of surgery.
Before the induction of anesthesia, a bispectral sensor (Aspect Medical Systems, Natick, MA, USA) was placed on the right side of the forehead, and an electroencephalogram (EEG) recording and Bispectral index (BIS) were recorded from the time of induction to the end of the surgery every 30 minutes. The BIS is based on an analysis of the EEG and represents the depth of anesthesia as a single value. The BIS ranges from 0 (complete cortical EEG suppression) to 100 (awake), and an index between 45 and 60 has been recommended for successful general anesthesia13). The maximum and minimum value of BIS and BIS under 40 was checked during the surgery.
Volatile anesthesia was used for usual operations, but total intravenous anesthesia was used in the cases with intraoperative neurophysiological monitoring. We also checked the patient's blood pressure every 10 minutes, total fluid balance, total operation time, and estimated blood loss.
The patients were observed for delirious symptoms every day from postoperative day one until discharge. The elderly patients were recommended to discharge seven days after the surgery after checking wound healing, exercise and postoperative delirium. Hemoglobin, hematocrit, and electrolyte levels were measured on the operation day and on postoperative day 1, 2, and 3. A chest radiograph was taken after recovery from anesthesia and one day after the surgery.
Data are presented as the mean ± standard deviation (SD). A Student's t test, χ2 test, and multivariate logistic regression analysis were used for statistical analysis. All variables with a significance level of p<0.05 in the univariate analysis were included as independent variables in a forward stepwise regression method for the multivariate analysis. A value of p<0.05 was considered statistically significant.
The patient group included 32 males and 38 females, with an average age 70.1±5.8 years (range 60 to 85 years). There were 17 (24.3%) patients in Group A. Ten were diagnosed on the first postoperative day, five on the second postoperative day and two on the third postoperative day. The average age of the patients in Group A was 70.2±6.4 years, and the male to female ratio was 1 : 1.4 (7 : 10). The average age in Group B was 70.3±5.3 years, and the male to female ratio was 1 : 1.1 (25 : 28). The two groups exhibited no significant difference in either age or male to female ratio. The average number of education years in Group A and Group B was 6.2±5.3 years and 7.2±5.0 years, respectively, with no statistical significance. With regard to medical history, there was no statistical significance between the groups in hypertension, diabetes, and previous smoking habits (Table 1).
The results of the MMSE-K, CDR, and GDS tests of preoperative cognitive function in Group A were 19.1±5.4 (p<0.01), 0.9±0.6 (p<0.05), and 3.3±1.1 (p<0.01), which were significantly lower than those of Group B, 25.6±3.4, 0.5±0.2, and 2.1±0.7, respectively (Table 2).
The preoperative duration of hospitalization were 3.5±2.4 days in Group A and 5.0±3.8 days in Group B (p<0.05), showing a longer preoperative hospitalization associated significantly with a higher occurrence of delirium. Postoperative delirium occurred on 1.5±0.9 postoperative days (Table 1).
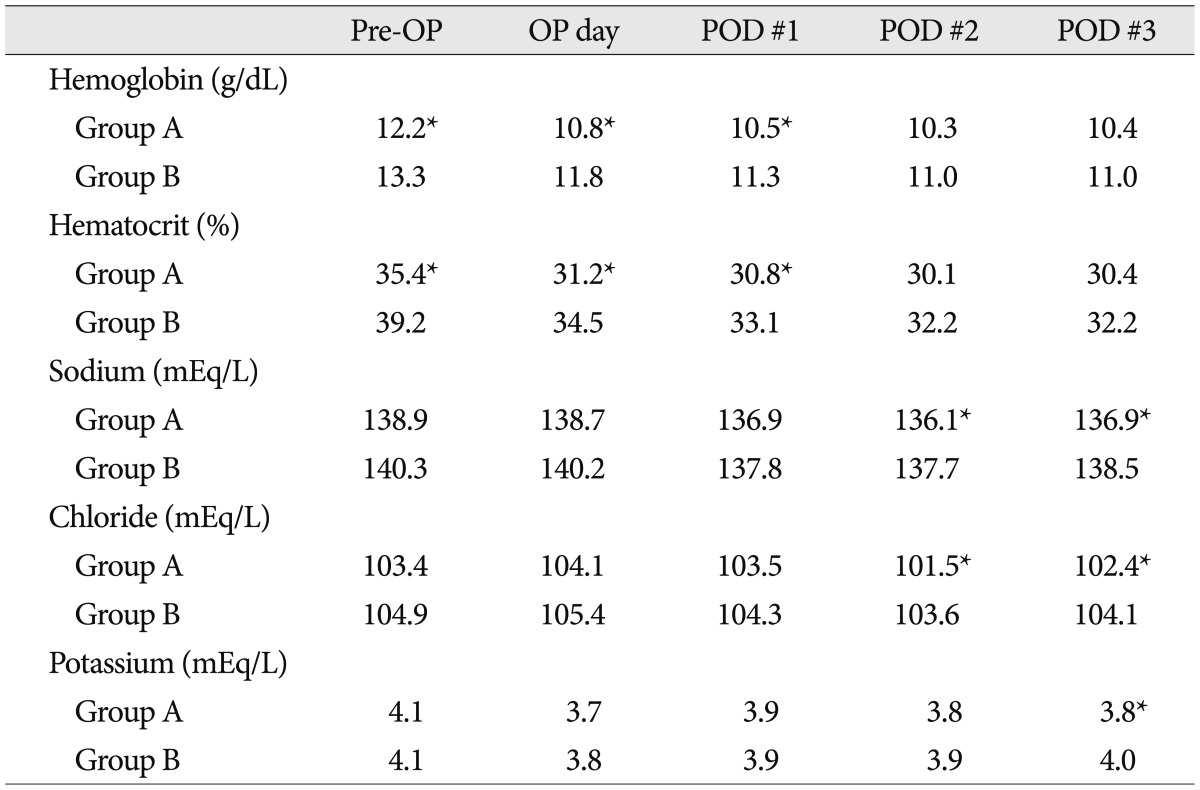
The hemoglobin (normal range : 11-15 g/dL) and hematocrit (normal range : 34-44%) levels were significantly lower in Group A at one day before surgery, immediately after surgery and postoperative day 1 (p<0.05). However, the data showed no significant difference after postoperative days 2 and 3. With regard to electrolytes, Na (normal range : 135-146 mEq/L) and Cl (normal range : 99-108 mEq/L) were significantly lower in Group A on postoperative day 2 and 3, and K (normal range : 3.5-5.3 mEq/L) was significantly lower in Group A only on postoperative day 3 (Table 3). But all the levels of electrolytes were within normal ranges.
The operation time in Group A (220.5±88.2 minutes) was significantly longer than that in Group B (165.0±68.7 minutes) (p<0.05). The intraoperative minimum value of BIS of Group A (30.2±6.8) was lower than that of Group B (35.4±5.6) (p<0.05). The number of BIS index under 40 was significantly higher in Group A, 5.1±3.1 times, whereas it was 2.5±2.2 times in Group B (p<0.01) (Table 4). The intraoperative fluid balance was 1317.2±791.5 mL in Group A and 849.3±472.2 mL in Group B (p<0.01), demonstrating a significantly larger positive fluid balance in Group A. The region of the spinal surgery (cervical or lumbar), anesthesia type, estimated blood loss, maximum BIS and blood pressure showed no significant relation with postoperative delirium (Table 1, 4).
Of the 18 subjects in Group A, six showed pulmonary congestion on the postoperative chest radiograph, and 13 of 53 subjects showed pulmonary congestion in Group B, which was not statistically significant (Table 1).
The multivariate analysis herein has found the preoperative GDS (95% CI OR, 1.20-25.60) and BIS measured intraoperatively under 40 (95% CI OR, 1.18-2.80) were associated with postoperative delirium (Table 5).
The pathophysiology of postoperative delirium is still unknown, although various pathophysiological mechanisms have been suggested in different situations. Cerejeira et al.3) suggested that cerebral effects of the systemic inflammatory response during the perioperative period, possibly aggravated by anticholinergic effects of drugs administered during this phase, might be responsible for postoperative delirium. Changes in the level of neurotransmitters, such as decreased cholinergic activity, increased dopaminergic activity, and decreased γ-aminobutyric acid-ergic activity, were also thought to be involved in delirium, but the interactions among neurotransmitters are complicated and incompletely understood9,33).
In many studies, postoperative delirium occurred in 10-60% of patients undergoing surgery, with a particularly high frequency in orthopedic pelvis surgery, aortic surgery and elderly patients5,20,24,25,33). One study reported the occurrence of delirium after brain surgery was 18.7%23). By contrast, the occurrence of postoperative delirium in degenerative lumbar spine surgery was reported to be relatively lower (13.6%), but in our study the incidence was considerably higher (24.3%)16). Depending on the symptoms, delirium can be divided into hyperactive, hypoactive and mixed subtypes10). The hyperactive subtype is easily detectable, but the hypoactive subtype is hard to differentiate from depression or dementia and often unrecognized10). The hypoactive subtype occurs more common in elderly patients than other types. Thus, in the present study, we interviewed the elderly subjects carefully to detect the hypoactive subtype and to observe subclinical symptoms of delirium. This effort might explain the higher incidence of hypoactive delirium detected herein compared to other studies.
Many studies have reported that old age is a significant risk factor for postoperative delirium10,18,21,24,33). However, this study found no group-to-group differences in the incidence of postoperative delirium, possibly because all the research subjects were older, reducing the likelihood of the role of age as a causative factor in their delirium. We found no postoperative delirium in those aged less than 60 years.
In a study of 797 hospitalized patients over 70 years of age, regardless of operation, Inouye et al.11) reported a delirium incidence of 16% and stated that the hospitalization itself could be a risk factor for delirium in the elderly. In our study, the period of the hospitalization before operation increases the possibility of postoperative delirium increased. As noted elsewhere, as postoperative delirium is very likely to lead to a chronic decline of cognitive function, efforts to prevent postoperative delirium must be very important29).
Cognitive function test results are not directly related to the existence of delirium and an abnormal result in a preoperative cognitive function test is not necessarily denoting delirium7). However, many studies have reported that preoperative cognitive impairment is a risk factor for postoperative delirium12,29,32). This is in agreement with the findings in the present study. The MMSE is widely utilized in research on preoperative cognitive function, and some studies reported that a low preoperative MMSE was a predictor of postoperative delirium2,6,29). Although the MMSE is a convenient and easy method to assess a patient's cognitive function, repeated MMSEs over short time intervals are not recommended because of the learning effect8,34). In this research, we utilized not only the MMSE in the cognitive function test but also the CDR and the GDS, and all three tests were found to be relevant to postoperative delirium in the univariate analysis. The GDS was the only correlated factor for postoperative delirium in the multivariate analysis. The GDS test was also more reliable than the MMSE, showing a strong association with clinical symptoms, behavior, and functional change and almost no association with educational and socioeconomic status. Thus, the GDS seems the most appropriate preoperative cognitive functional test27). As cognitive impairment is an important causative factor of delirium in the elderly, all patients with a high probability of postoperative delirium in a cognitive functional test should be advised beforehand about its occurrence10).
The frequency of exposure to deep anesthesia was reported to increase the incidence of postoperative delirium4). According to our data, a minimum BIS and the number of BIS <40 were significant risk factors. The multivariate analysis showed that the occurrence of postoperative delirium increased significantly in accordance with the increased number of BIS index <40, indicating much more frequent intraoperative deep anesthetic exposure. The BIS <40 is known to be associated with an increase of EEG burst suppression and theta activity, which is a typical EEG patterns observed in delirium patients, and is assumed to have affected the incidence of postoperative delirium25,28). Thus, to reduce postoperative delirium, it may be helpful to monitor and maintain the intraoperative BIS index level above 40 with the cooperation of an anesthesiologist.
Kawaguchi et al.15) suggested that low hemoglobin could reduce the oxygen supply to the brain, leading to delirium. Marcantonio et al.20) considered the low hematocrit in perioperative setting could cause insult in central nervous system and develop delirium, and recommended that transfusion should be performed to keep the hematocrit level over 30% in high risk patients. This idea is consistent with the findings of our study. Although the hemoglobin and hematocrit levels were all within the normal range in both groups, Group A showed significantly lower postoperative levels than Group B. The findings are seemed to be partially related with the significantly lower hemoglobin and hematocrit levels in Group A. Therefore, a meticulous intraoperative bleeding control and perioperative transfusion to maintain hemoglobin/hematocrit levels seem to be helpful to reduce postoperative delirium.
An electrolyte imbalance is also known as a cause of postoperative delirium6). In the present study, Na and Cl were significantly lower in Group A on postoperative days 2 and 3, and K was significantly lower in Group A on postoperative day 3 compared to Group B. However, all the levels were within the normal range, embedding a difficult to make a clinical significance. This study also showed that an increase in the intraoperative fluid positive balance was significantly related to postoperative delirium. This is likely because fluid overload is associated with hemoglobin and hematocrit levels and the electrolyte balance.
Old age is known to be a vital factor in postoperative delirium. Although the enrolled patients were all from the old age group (>60), they could be still affected by age-related factors, but the number of patients was not enough to detect such influences10,18,21,24,33). Further studies will be needed to examine a larger number of old-aged patients to detect more detailed age-specific effects. Postoperative pain killers are known to be associated with postoperative delirium17,19). Opioid analgesics were also reported to induce postoperative delirium9,30,33). Although we used PCA containing fentanyl postoperatively, we could not analyze its effect on postoperative delirium because all the patients except two received PCA. Further studies should be necessary to elucidate the role postoperative medication in the development of postoperative delirium.
Based on our data, it seems that spinal surgeries can be associated with a considerably high occurrence of postoperative delirium. Although this study identified several new risk factors for delirium, it seems that spinal surgeries do not differ greatly from other surgeries in terms of risk factors and mechanisms.
With spinal surgeries, in contrast to other high-risk operations involving the brain or other vital organs, it is difficult for both the doctor and the patient to anticipate postoperative mental functional problems. Therefore, it must be difficult situation to accept for both doctors and patient's family if delirium develops after spinal surgery. A proper understanding of the risk factors for postoperative delirium would be helpful to prevent postoperative delirium and to minimize medicolegal problems.
References
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Washington, DC: American Psychiatric Association;2013.
2. Boos GL, Soares LF, Oliveira Filho GR. [Postoperative cognitive dysfunction : prevalence and associated factors]. Rev Bras Anestesiol. 2005; 55:517–524. PMID: 19468642.
3. Cerejeira J, Firmino H, Vaz-Serra A, Mukaetova-Ladinska EB. The neuroinflammatory hypothesis of delirium. Acta Neuropathol. 2010; 119:737–754. PMID: 20309566.

4. Chan MT, Cheng BC, Lee TM, Gin T. CODA Trial Group. BIS-guided anesthesia decreases postoperative delirium and cognitive decline. J Neurosurg Anesthesiol. 2013; 25:33–42. PMID: 23027226.

5. Deiner S, Silverstein JH. Postoperative delirium and cognitive dysfunction. Br J Anaesth. 2009; 103(Suppl 1):i41–i46. PMID: 20007989.

6. Elie M, Cole MG, Primeau FJ, Bellavance F. Delirium risk factors in elderly hospitalized patients. J Gen Intern Med. 1998; 13:204–212. PMID: 9541379.

7. Fayers PM, Hjermstad MJ, Ranhoff AH, Kaasa S, Skogstad L, Klepstad P, et al. Which mini-mental state exam items can be used to screen for delirium and cognitive impairment? J Pain Symptom Manage. 2005; 30:41–50. PMID: 16043006.

8. Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975; 12:189–198. PMID: 1202204.
9. Gaudreau JD, Gagnon P. Psychotogenic drugs and delirium pathogenesis : the central role of the thalamus. Med Hypotheses. 2005; 64:471–475. PMID: 15617851.

11. Inouye SK, Foreman MD, Mion LC, Katz KH, Cooney LM Jr. Nurses' recognition of delirium and its symptoms : comparison of nurse and researcher ratings. Arch Intern Med. 2001; 161:2467–2473. PMID: 11700159.
12. Jankowski CJ, Trenerry MR, Cook DJ, Buenvenida SL, Stevens SR, Schroeder DR, et al. Cognitive and functional predictors and sequelae of postoperative delirium in elderly patients undergoing elective joint arthroplasty. Anesth Analg. 2011; 112:1186–1193. PMID: 21415433.

13. Johansen JW. Update on bispectral index monitoring. Best Pract Res Clin Anaesthesiol. 2006; 20:81–99. PMID: 16634416.

14. Kang Y, Na DL, Hahn S. A validity study on the Korean Mini-Mental State Examination (K-MMSE) in dementia patients. J Korean Neurol Assoc. 1997; 15:300–308.
15. Kawaguchi Y, Kanamori M, Ishihara H, Abe Y, Nobukiyo M, Sigeta T, et al. Postoperative delirium in spine surgery. Spine J. 2006; 6:164–169. PMID: 16517388.

16. Lee JK, Park YS. Delirium after spinal surgery in Korean population. Spine (Phila Pa 1976). 2010; 35:1729–1732. PMID: 20150830.

17. Leung JM, Sands LP, Paul S, Joseph T, Kinjo S, Tsai T. Does postoperative delirium limit the use of patient-controlled analgesia in older surgical patients? Anesthesiology. 2009; 111:625–631. PMID: 19672166.

18. Litaker D, Locala J, Franco K, Bronson DL, Tannous Z. Preoperative risk factors for postoperative delirium. Gen Hosp Psychiatry. 2001; 23:84–89. PMID: 11313076.

19. Lynch EP, Lazor MA, Gellis JE, Orav J, Goldman L, Marcantonio ER. The impact of postoperative pain on the development of postoperative delirium. Anesth Analg. 1998; 86:781–785. PMID: 9539601.

20. Marcantonio ER, Goldman L, Orav EJ, Cook EF, Lee TH. The association of intraoperative factors with the development of postoperative delirium. Am J Med. 1998; 105:380–384. PMID: 9831421.

21. Morimoto Y, Yoshimura M, Utada K, Setoyama K, Matsumoto M, Sakabe T. Prediction of postoperative delirium after abdominal surgery in the elderly. J Anesth. 2009; 23:51–56. PMID: 19234823.

22. Morris JC. The Clinical Dementia Rating (CDR) : current version and scoring rules. Neurology. 1993; 43:2412–2414. PMID: 8232972.
23. Oh YS, Kim DW, Chun HJ, Yi HJ. Incidence and risk factors of acute postoperative delirium in geriatric neurosurgical patients. J Korean Neurosurg Soc. 2008; 43:143–148. PMID: 19096622.

24. Parikh SS, Chung F. Postoperative delirium in the elderly. Anesth Analg. 1995; 80:1223–1232. PMID: 7762856.

25. Plaschke K, Fichtenkamm P, Schramm C, Hauth S, Martin E, Verch M, et al. Early postoperative delirium after open-heart cardiac surgery is associated with decreased bispectral EEG and increased cortisol and interleukin-6. Intensive Care Med. 2010; 36:2081–2089. PMID: 20689917.

26. Reisberg B, Ferris SH, de Leon MJ, Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry. 1982; 139:1136–1139. PMID: 7114305.

27. Reisberg B, Jamil IA, Khan S, Monteiro I, Torossian C, Ferris S, et al. Staging dementia. In : Abou-Saleh M, Katona C, Kumar A, editors. Principles and Practice of Geriatric Psychiatry. ed 33. London: John Wiley & Sons, Ltd;p. 162–169.
28. Rundshagen I, Hardt T, Cortina K, Pragst F, Fritzsche T, Spies C. Narcotrend-assisted propofol/remifentanil anaesthesia vs clinical practice : does it make a difference? Br J Anaesth. 2007; 99:686–693. PMID: 17704091.

29. Saczynski JS, Marcantonio ER, Quach L, Fong TG, Gross A, Inouye SK, et al. Cognitive trajectories after postoperative delirium. N Engl J Med. 2012; 367:30–39. PMID: 22762316.

30. Schuurmans MJ, Duursma SA, Shortridge-Baggett LM, Clevers GJ, Pel-Littel R. Elderly patients with a hip fracture : the risk for delirium. Appl Nurs Res. 2003; 16:75–84. PMID: 12764718.
31. Silverstein JH, Timberger M, Reich DL, Uysal S. Central nervous system dysfunction after noncardiac surgery and anesthesia in the elderly. Anesthesiology. 2007; 106:622–628. PMID: 17325520.

32. Smith PJ, Attix DK, Weldon BC, Greene NH, Monk TG. Executive function and depression as independent risk factors for postoperative delirium. Anesthesiology. 2009; 110:781–787. PMID: 19326492.

33. Steiner LA. Postoperative delirium. Part 1 : pathophysiology and risk factors. Eur J Anaesthesiol. 2011; 28:628–636. PMID: 21785356.
34. Tombaugh TN, McIntyre NJ. The mini-mental state examination : a comprehensive review. J Am Geriatr Soc. 1992; 40:922–935. PMID: 1512391.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download