Abstract
Objective
The purpose of this study was to investigate the clinical features and outcomes of pediatric cavernous malformation (CM) in the central nervous system.
Methods
Twenty-nine pediatric patients with supratentorial CM underwent microsurgical excision. In selected cases, transparent tubular retractor system (TTRS) was used to reduce retraction injury and intraoperative neuromonitoring (IONM) was held to preserve functioning cortex. Patients' demographics and symptoms were reviewed and surgical outcomes were discussed.
Results
The main initial clinical manifestations included the following : seizures (n=13, 45%), headache (n=7, 24%), focal neurological deficits (n=3, 10%), and an incidental finding (n=6, 21%). Overt hemorrhage was detected in 7 patients (24%). There were 19 children (66%) with a single CM and 10 (34%) children with multiple CMs. In 7 cases with deep-seated CM, we used a TTRS to minimize retraction. In 9 cases which location of CM was at eloquent area, IONM was taken during surgery. There was no major morbidity or mortality after surgery. In the 29 operated children, the overall long-term results were satisfactory : 25 (86%) patients had no signs or symptoms associated with CMs, 3 had controllable seizures, and 1 had mild weakness.
Conclusion
With the assistance of neuronavigation systems, intraoperative neuromonitoring, and TTRS, CMs could be targeted more accurately and excised more safely. Based on the satisfactory seizure outcome achieved, complete microsurgical excision in children is recommended for CMs presenting with seizures but removal of hemosiderin-stained areas seems to be unnecessary.
Cerebral cavernous malformations (CMs), also termed as cavernous angiomas, cavernous hemangiomas, or cavernomas, are vascular malformations consisting of thin hyalinized vascular channels without interposed brain tissue. In the pediatric age group, CMs have been reported to be one of the main causes of brain hemorrhage along with ruptured arteriovenous malformations (AVMs)19,20). In addition, the risk for seizures in patients with a previous history of epilepsy due to CMs is 2 to 4 times higher than that in patients without a previous history of epilepsy18,23). Patients with CMs who are under the age of 40 years are 5.6 times more likely to have a lifelong disability due to seizures than older patients24), especially when the seizures have started during childhood1,2).
Treatment options may vary depending on the patient's presenting symptoms and the location of CMs. Because radiosurgery for CMs does not seem to have a real advantage over conservative or medical therapy, surgical resection of the whole lesion seems to be the ideal treatment for symptomatic lesions1). Recent advances in neuronavigation systems have made targeting of small-sized CMs and approaching the deep-seated regions easier. Early diagnosis and complete resection of lesions can prevent neurological deterioration from repeated bleeding. Furthermore, in patients presenting with seizures, early surgical resection would prevent the epileptogenic focus to enlarge and involve surrounding brain tissue which might include the hemosiderin border. However, seizure outcomes with respect to resection of the hemosiderin border still remains unclear in children with supratentorial CMs3).
The purpose of this study was to investigate the clinical features and outcomes of pediatric CMs treated with surgical resection by using neuronavigation systems, intraoperative neuromonitoring, and TTRS.
We retrospectively reviewed the clinic-radiological data of 29 children (under 18 years) who underwent surgical resection of supratentorial CMs between January 2004 and June 2012. The diagnosis of CM was confirmed pathologically in all patients. Preoperative MRI was available in all patients, and lesion location, number of lesions, and whether the lesions were located in an eloquent area or not were evaluated. We treated all patients with craniotomy and lesionectomy. There were 10 (34%) patients with multiple lesions, and lesionectomy was selected based on the correlation of the lesion with the presenting symptoms. If lesion localization was not achievable, the lesion with an overt hemorrhage or the largest lesion that caused a mass effect was removed. In patients with acute symptomatic hemorrhage, we waited until the liquefaction of hematoma and improvement of neurological deficit occurred unless the symptoms worsen or neurological deficits progress. We did not remove the hemosiderin-stained area surrounding the core lesion; we only removed the vascular malformation.
We used intraoperative ultrasonography in the earlier cases (n=4). A frameless navigation system (BrainLab, Feldkirchen, Germany) has been applied in 24 patients since 2006. The navigation system helped us to choose the shortest pathway to a deeply located lesion and to remove the lesion precisely with mini-craniotomy (Fig. 1). We used the transparent tubular retraction system (TTRS) in 7 patients who had the lesion in a deep-seated region to minimize the incidence of retraction injury to the normal parenchyma and secure an adequate working space for gross removal of the lesion (Fig. 2). We have previously reported the feasibility of TTRS in patients with deep-seated tumors13). When the lesion was located in an eloquent area, especially in the primary motor cortex or descending pyramidal tracts, intraoperative monitoring of motor evoked potentials (MEP) and somatosensory evoked potentials (SSEPs) were performed. In all cases, immediate postoperative CT was performed to detect hemorrhagic complications. MRI was taken postoperatively to detect residual lesions as well as late complications. In seizure patients, residual hemosiderin-stained areas were assessed on MRI for confirming the role of hemosiderin in seizure patients.
Neurological examinations were performed pre- and post-operatively. Extended Glasgow outcome scale (GOS-E) was used to assess surgical outcomes31). In thirteen patients who presented with seizures, Engel's classification was used to measure the surgical outcome, including the functional status of a seizure patient9). The average follow-up period was 27 months (range, 1 month to 8 years). In seizure patients, the average follow-up period was 37.7 months. Except for one 14-month-old boy who was lost to follow-up from 3 months after surgery, all other seizure patients were followed up for at least 20 months.
Of the 29 children with supratentorial CMs, 18 were males. Their age at the time of surgery varied from 9 months to 18 years, and the mean age was 9.4 years. The most common presenting symptom was seizures; 13 patients (45%) presented with seizures (3 cases of simple partial seizures and 10 cases of generalized tonic-clonic seizures). Patients' demographics who presented with seizure are shown in Table 1. The second most common symptom that was reported by 8 patients (28%) was mild neurological symptoms such as headache, dizziness, nausea and vomiting. Only two patients (7%) presented with focal neurological deficits including weakness and delayed speech. In 6 patients (21%), CMs were incidentally detected on MRI, which was mostly performed after minor head trauma. Regardless of the severity of symptoms, variable amount of overt hemorrhage was detected in 7 patients (24%) during their initial imaging study. Evaluation of the lesion number on MRI revealed that 19 patients (66%) had a single lesion, and 10 patients (34%) showed multiple lesions. Evaluation of the lesion location on MRI revealed that in 26 patients CMs were detected only in the supratentorial area (90%) and in 3 patients, CMs were detected in both the supra- and infratentorial area (10%). In 10 cases (34%), CMs were located in the eloquent areas.
Post-operative imaging showed that 26 of 29 patients had complete resection of the target lesion, which accounts for 90% of the cases. A residual lesion was found in 3 patients. One of these 3 patients had a lesion in the left precentral gyrus and had subtotal resection due to change of monitoring during surgery. The other two had a deep-seated lesion in the caudate nucleus, that one of them seemed to be removed totally on visual inspection, but a residual lesion was detected on postoperative MRI and the other had subtotal resection due to change of evoked potential during surgery. Except for these 3 (10%) patients, complete resection was performed in all patients and was confirmed by postoperative MRI. Of the 13 patients who presented with seizures, all had hemosiderin-stained areas surrounding the lesion.
There was no perioperative mortality or severe morbidity that showed worse neurological deficits than pre-operative neurological status; however, some minor complications occurred during the follow-up. 3 (10%) patients had transient weakness or sensory changes immediately after surgery, but they were recovered completely. Assessment of GOS-E revealed one patient with a score of 6 and another with a score of 7, representing mild disabilities. Their symptoms were constant after surgery compared to their symptoms before surgery.
Of the 13 patients who presented with seizures, 10 patients did not develop seizures postoperatively during follow-ups of 20-99 months (Engel class IA). Two patients developed generalized tonic-clonic (GTC) seizures 2 months after surgery, but there was no seizure thereafter without antiepileptic medication (Engel class IC). The remaining one patient with a lesion in the right frontal lobe presented with GTC seizures at the time of diagnosis; he underwent surgery and did not experience seizure for 18 months. He stopped taking antiepileptics, but after 14 months he had simple partial seizures (Engel class IB). He restarted antiepileptics. Assessment of GOS-E revealed all of the 13 patients with score 8 (Table 2).
A 15-year-old boy visited the emergency department with an ongoing headache that had aggravated since 2 weeks. He had no underlying diseases, and there was no history of trauma. He underwent CT and hemorrhage was detected in the right parieto-occipital lobe. Brain MRI was performed to evaluate the underlying vascular malformations, and multiple popcorn-like lesions were detected (Fig. 2A). Surgical removal of hematoma and accompanying lesion was planned. With the assistance of the frameless navigation system (BrainLab), a trajectory for approaching the lesion was planned. A small-sized craniotomy was performed in the parietal area, and the TTRS was inserted into the lesion with the assistance of the navigation system. By retraction with the TTRS, we could reach the deep lesion without excessive retraction and secure an adequate surgical working space for removal of the lesion (Fig. 2B). Adequate working space for bipolar forceps and suction was provided with minimal damage to the parenchyma. We sucked out the old hematoma around the lesion, and complete removal of the lesion was performed. Only dissection around the margin of the CM was performed and the surrounding hemosiderin-stained area was not removed in effort. The patient had a mild headache after surgery, but there was no other complications. MRI performed after 3 months showed no residual lesion, but hemosiderin deposits around the lesion were observed. Also, there were no changes in the other multiple lesions (Fig. 2C).
A 17-year-old girl had involuntary movement of her left hand since one year before her visit to our clinic. She was taking AEDs, but 2 more seizures occurred in a similar manner. She underwent brain MRI for evaluating the underlying cause of her symptoms and a popcorn-like lesion was detected, which strongly represents a CM. The CM was located in the right precentral gyrus (Fig. 3A). The lesion was found to be in the eloquent area that plays a role in the motor functions of the left hemisphere. She had recurrent seizures even though she was taking proper AEDs. We planned to remove the lesion that may be responsible for her symptoms. Under assistance of the navigation system, the location of the lesion was carefully determined. Since the lesion was in the precentral gyrus, intra-operative neuromonitoring of MEPs and SSEPs was performed. A careful craniotomy was performed with the assistance of the navigation system. The lesion was located immediately below the cortex, and we could easily locate the lesion by performing a minimal corticotomy. Careful removal of the lesion was accomplished, and no changes were reported in the MEPs and SSEPs during surgery. She had no motor weakness after surgery, but numbness developed in her left 4th and 5th fingers. She was seizure-free for 6 more months with taking AEDs, after which she stopped the medication and is still seizure-free. She underwent an MRI one year after surgery and no residual lesion was detected; however, hemosiderin deposition was observed (Fig. 3B).
Cerebral cavernous malformations (CMs) are vascular malformations consisting of thin hyalinized vascular channels without interposed brain tissue. CMs have been known to be present in about 0.4-0.8% of the population18); and one-fourth of CMs occur in the pediatric population7).
CMs are known to have a frequency of symptomatic hemorrhage ranging from 0.1% to 2.7% per lesion-year and 0.013% to 16.5% per patient-year29). In children, CM-related hemorrhagic events are approximately 2-3 times more frequent than in adults1). CMs have been reported to be one of the main causes of brain hemorrhage along with ruptured arteriovenous malformations (AVMs)19,20) in the pediatric age group. Considering the increased risk of re-hemorrhage, surgery should be performed in all symptomatic CMs that are located in eloquent regions because it appears to be a safe option under the modern monitoring conditions32). Clinical results in children who were treated with surgical therapy for CMs of the CNS are generally excellent or good in majority of pediatric series8,11,16,17,19,25). In addition, surgery led to the complete cure of epilepsy (i.e., discontinuation of anticonvulsant therapy) in 31% of the children with preoperative seizures, while better therapeutic control of seizures was achieved in more than 48% of cases1). Given the hemosiderin-related epileptogenesis26,30), it is generally agreed that the hemosiderin-stained areas or gliotic tissue should be removed in order to achieve complete relief from seizures, and complete removal of the hemosiderin-stained area may improve the long-term outcome4,5,10,28,31,34). However, even without performing total removal of hemosiderin-stained areas, we could achieve a satisfactory seizure control outcome. It assumed that additional resection of hemosiderin-stained areas appears to be unnecessary in children. We have previously reported that early surgical removal of superficial CMs or of CMs that are not critically located but do cause seizures may provide a real curative treatment for epilepsy33), thus preventing psychosocial disability in patients on long-term medical therapy and avoiding the risk of neurological deficits due to growth and hemorrhage in the CMs. In children, the growth behavior of CMs is more aggressive than in adults. Also, the risk of hemorrhage or frequency of developing seizures is higher than that of adults1,15,18,21). Considering that the hemosiderin rim would grow larger and take a role in epileptogenesis, early removal of CM with or without resection of the hemosiderin rim would be helpful especially in pediatric group, too. In addition, children have enhanced capacity for brain plasticity compared to adults, and they have capacity to recover from brain injuries or radical surgery14). Therefore, more aggressive surgical resection of CMs would be required in pediatric patients.
In the present study, 10 patients presented with multiple CMs. In cases of multiple CMs, surgery of the symptomatic lesion should be performed. In the illustrated case of this study shows the benefit of early surgery under modern monitoring conditions. Though there were some residuals left, regarding its size and locations, we believe that early surgery prevented the child to have disastrous neurological deteriorations from rebleeding of the CMs. Though multiple CMs may occur in more than 30% of sporadic CMs12,22), autosomal dominant familial CMs should be always considered. The hemorrhagic risk of familial CMs is believed to be higher than that of sporadic CMs6). Although genetic testing was not performed in the present study, this may have substantial benefits in managing patients with multiple CMs.
Today, the widespread use of MRIs, functional MRIs, intraoperative neuromonitoring, neuronavigation systems, brain mapping, and awake craniotomy can minimize the postoperative morbidity in patients with CMs in the most eloquent locations27,32). For an incidental CM in an eloquent location, surgery is not advisable due to a still significant risk of postoperative deterioration32). In our series, with the assistance of neuronavigation system, TTRS, and also intraoperative neuromonitoring, a satisfactory outcome and seizure control was achieved in all patients with CMs. Since patients with cerebral CMs located in an eloquent region who present with seizures can be cured with surgical treatment32), detailed evaluation of the lesions that correlate with the presenting symptoms and vigilant surgical resection must be considered.
We retrospectively analyzed a surgical cohort of children with supratentorial CMs, and thus, our findings are limited by selection bias and the inherent biases associated with a retrospective study. Moreover, we could not demonstrate the statistical superiority of neuronavigation or TTRS-assisted surgery over conventional surgery because the number of cases enrolled was relatively small and most patients had good clinical outcomes. Unfortunately, genetic differences in multiple CMs were not evaluated in the study that we could not show natural courses of CMs with genetic alterations.
For a symptomatic solitary CM, the treatment of choice is complete microsurgical excision preceded by careful anatomical and functional evaluation. If the lesion is located in the eloquent area, surgical intervention may be very challenging for surgeons. With the assistance of neuronavigation systems, intraoperative neuromonitoring, and TTRS, the lesion could be targeted more accurately and excised more safely with minimization of normal tissue damages. Furthermore, based on the satisfactory seizure outcomes achieved, early resection is recommended for CMs presenting with seizures but additional resection of hemosiderin-stained areas appears to be unnecessary in children.
References
1. Acciarri N, Galassi E, Giulioni M, Pozzati E, Grasso V, Palandri G, et al. Cavernous malformations of the central nervous system in the pediatric age group. Pediatr Neurosurg. 2009; 45:81–104. PMID: 19307743.

2. Aiba T, Tanaka R, Koike T, Kameyama S, Takeda N, Komata T. Natural history of intracranial cavernous malformations. J Neurosurg. 1995; 83:56–59. PMID: 7782850.

3. Baumann CR, Acciarri N, Bertalanffy H, Devinsky O, Elger CE, Lo Russo G, et al. Seizure outcome after resection of supratentorial cavernous malformations : a study of 168 patients. Epilepsia. 2007; 48:559–563. PMID: 17346251.

4. Baumann CR, Schuknecht B, Lo Russo G, Cossu M, Citterio A, Andermann F, et al. Seizure outcome after resection of cavernous malformations is better when surrounding hemosiderin-stained brain also is removed. Epilepsia. 2006; 47:563–566. PMID: 16529622.

5. Casazza M, Broggi G, Franzini A, Avanzini G, Spreafico R, Bracchi M, et al. Supratentorial cavernous angiomas and epileptic seizures : preoperative course and postoperative outcome. Neurosurgery. 1996; 39:26–32. discussion 32-34. PMID: 8805137.
6. Cohen-Gadol AA, Jacob JT, Edwards DA, Krauss WE. Coexistence of intracranial and spinal cavernous malformations : a study of prevalence and natural history. J Neurosurg. 2006; 104:376–381. PMID: 16572649.

7. Del Curling O Jr, Kelly DL Jr, Elster AD, Craven TE. An analysis of the natural history of cavernous angiomas. J Neurosurg. 1991; 75:702–708. PMID: 1919691.

8. Di Rocco C, Iannelli A, Tamburrini G. Cavernomas of the central nervous system in children. A report of 22 cases. Acta Neurochir (Wien). 1996; 138:1267–1274. discussion 1273-1274. PMID: 8980728.
9. Engel J Jr, Levesque MF, Shields WD. Surgical treatment of the epilepsies : presurgical evaluation. Clin Neurosurg. 1992; 38:514–534. PMID: 1537201.
10. Ferroli P, Casazza M, Marras C, Mendola C, Franzini A, Broggi G. Cerebral cavernomas and seizures : a retrospective study on 163 patients who underwent pure lesionectomy. Neurol Sci. 2006; 26:390–394. PMID: 16601930.

11. Herter T, Brandt M, Szüwart U. Cavernous hemangiomas in children. Childs Nerv Syst. 1988; 4:123–127. PMID: 3396017.

12. Hsu F, Rigamonti D, Huhn SL. Epidemiology of cavernous malformations. In : Awad IA, Barrow DL, editors. Cavernous malformations. Park Ridge: American Association of Neurological Surgeons;1993. p. 13–23.
13. Jo KI, Chung SB, Jo KW, Kong DS, Seol HJ, Shin HJ. Microsurgical resection of deep-seated lesions using transparent tubular retractor : pediatric case series. Childs Nerv Syst. 2011; 27:1989–1994. PMID: 21779977.

15. Kawagishi J, Suzuki M, Kayama T, Yoshimoto T. Huge multilobular cavernous angioma in an infant : case report. Neurosurgery. 1993; 32:1028–1030. discussion 1030-1031. PMID: 8327078.
16. Lena G, Ternier J, Paz-Paredes A, Scavarda D. [Central nervous system cavernomas in children]. Neurochirurgie. 2007; 53(2-3 Pt 2):223–237. PMID: 17507057.
17. Mazza C, Scienza R, Beltramello A, Da Pian R. Cerebral cavernous malformations (cavernomas) in the pediatric age-group. Childs Nerv Syst. 1991; 7:139–146. PMID: 1878867.

18. Moriarity JL, Wetzel M, Clatterbuck RE, Javedan S, Sheppard JM, Hoenig-Rigamonti K, et al. The natural history of cavernous malformations : a prospective study of 68 patients. Neurosurgery. 1999; 44:1166–1171. discussion 1172-1173. PMID: 10371615.

19. Mottolese C, Hermier M, Stan H, Jouvet A, Saint-Pierre G, Froment JC, et al. Central nervous system cavernomas in the pediatric age group. Neurosurg Rev. 2001; 24:55–71. discussion 72-73. PMID: 11485241.

20. Papadias A, Taha A, Sgouros S, Walsh AR, Hockley AD. Incidence of vascular malformations in spontaneous intra-cerebral haemorrhage in children. Childs Nerv Syst. 2007; 23:881–886. PMID: 17450369.

21. Pozzati E, Giuliani G, Nuzzo G, Poppi M. The growth of cerebral cavernous angiomas. Neurosurgery. 1989; 25:92–97. PMID: 2755587.

22. Robinson JR, Awad IA. Clinical spectrum and natural course. In : Awad IA, Barrow DL, editors. Cavernous malformations. Park Ridge: American Association of Neurological Surgeons;1993. p. 25–36.
23. Robinson JR, Awad IA, Little JR. Natural history of the cavernous angioma. J Neurosurg. 1991; 75:709–714. PMID: 1919692.

24. Robinson JR Jr, Awad IA, Magdinec M, Paranandi L. Factors predisposing to clinical disability in patients with cavernous malformations of the brain. Neurosurgery. 1993; 32:730–735. discussion 735-736. PMID: 8492847.

25. Sakai N, Yamada H, Nishimura Y, Shirakami S, Futamura A, Andoh T. Intracranial cavernous angioma in the 1st year of life and a review of the literature. Childs Nerv Syst. 1992; 8:49–52. PMID: 1576609.

26. Ueda Y, Willmore LJ, Triggs WJ. Amygdalar injection of FeCl3 causes spontaneous recurrent seizures. Exp Neurol. 1998; 153:123–127. PMID: 9743573.
27. Wang CC, Liu A, Zhang JT, Sun B, Zhao YL. Surgical management of brain-stem cavernous malformations : report of 137 cases. Surg Neurol. 2003; 59:444–454. discussion 454. PMID: 12826334.

28. Wang X, Tao Z, You C, Li Q, Liu Y. Extended resection of hemosiderin fringe is better for seizure outcome : a study in patients with cavernous malformation associated with refractory epilepsy. Neurol India. 2013; 61:288–292. PMID: 23860150.

29. Washington CW, McCoy KE, Zipfel GJ. Update on the natural history of cavernous malformations and factors predicting aggressive clinical presentation. Neurosurg Focus. 2010; 29:E7. PMID: 20809765.

30. Willmore LJ, Triggs WJ. Iron-induced lipid peroxidation and brain injury responses. Int J Dev Neurosci. 1991; 9:175–180. PMID: 2058418.

31. Wilson JT, Pettigrew LE, Teasdale GM. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale : guidelines for their use. J Neurotrauma. 1998; 15:573–585. PMID: 9726257.

32. Wostrack M, Shiban E, Harmening K, Obermueller T, Ringel F, Ryang YM, et al. Surgical treatment of symptomatic cerebral cavernous malformations in eloquent brain regions. Acta Neurochir (Wien). 2012; 154:1419–1430. PMID: 22739772.

33. Yeon JY, Kim JS, Choi SJ, Seo DW, Hong SB, Hong SC. Supratentorial cavernous angiomas presenting with seizures : surgical outcomes in 60 consecutive patients. Seizure. 2009; 18:14–20. PMID: 18656386.

34. Zevgaridis D, van Velthoven V, Ebeling U, Reulen HJ. Seizure control following surgery in supratentorial cavernous malformations : a retrospective study in 77 patients. Acta Neurochir (Wien). 1996; 138:672–677. PMID: 8836281.

Fig. 1
Thin section MRI was taken preoperatively and registered to the intraoperative neuronavigation system. With help of neuronavigation system, planning of the optimal trajectory to the target lesion could be easily achieved and margins of the lesion were assumed during surgery.
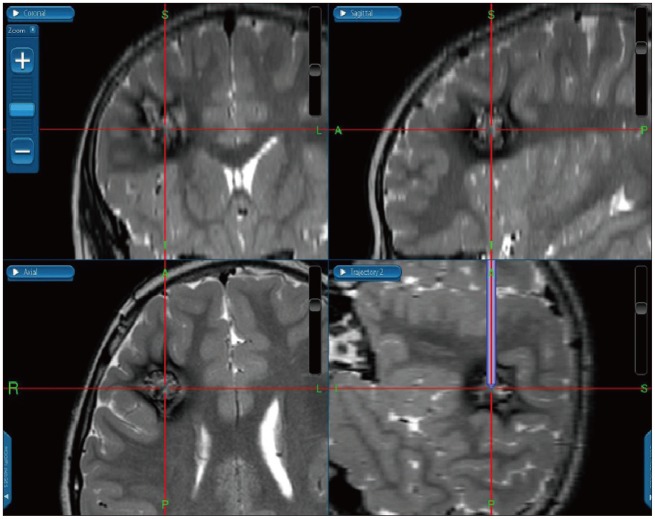
Fig. 2
Preoperative MRI of 15-year-old boy showed multiple dark signal structures including right parieto-occipital area (A). Transparent tubular retraction system (TTRS) was inserted via minimal corticotomy. Without wide cortical incision and excessive parenchymal retraction, competent surgical view and flexible working space through TTRS was achieved (B). MRI performed at 3 month after surgery did not show a residual lesion, but dark signal intensity was observed in T2WI, which indicates that the hemosiderin-stained area was left behind (C).
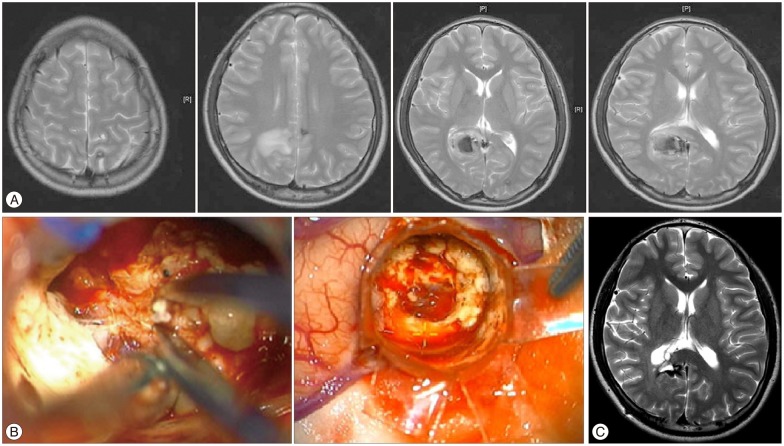
Fig. 3
Preoperative MRI of 17-year-old girl showed popcorn-like structure in the right precentral gyrus (A). One year after resection of the lesion in the precentral gyrus, there was no residual cavernous malformation, but there was hemosiderin deposition (B).





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download