Abstract
Objective
Moyamoya disease (MMD) is a chronic cerebrovascular occlusive disease of unknown etiology. In addition, the neurocognitive impairment of adults with MMD is infrequently reported and, to date, has not been well described. We attempted to determine both the neurocognitive profile of adult moyamoya disease and whether a superficial temporal artery-middle cerebral artery (STA-MCA) anastomosis can improve the neurocognitive impairment in exhibiting hemodynamic disturbance without stroke.
Methods
From September 2010 through November 2012, 12 patients with angiographically diagnosed MMD underwent STA-MCA anastomosis for hemodynamic impairment. Patients with hypoperfusion and impaired cerebrovascular reserve (CVR) capacity but without evidence of ischemic stroke underwent a cognitive function test, the Seoul Neuropsychological Screening Battery (SNSB). Five patients agreed to undergo a follow-up SNSB test. Data from preoperative and postoperative neurocognitive function tests were compared and analyzed.
Results
Five of 12 patients were enrolled. The median age was 45 years (range, 24-55 years). A comparison of preoperative to postoperative status of SNSB, memory domain, especially delayed recall showed significant improvement. Although most of the domains showed improvement after surgery, the results were not statistically significant.
Conclusion
In our preliminary study, large proportions of adult patients with MMD demonstrate disruption of cognitive function. This suggests the possibility of chronic hypoperfusion as a primary cause of the neurocognitive impairment. When preoperative and postoperative status of cognitive function was compared, memory domain showed remarkable improvement. Although further study is needed, neurocognitive impairment may be an indication for earlier intervention with reperfusion procedures that can improve cognitive function.
Moyamoya disease (MMD) is a rare bilateral cerebrovascular occlusive disorder of unknown etiology; it causes hemodynamic dysfunction that leads to neurocognitive impairment18). Usually, MMD is diagnosed after a cerebral hemorrhage in adults and a transient ischemic attack in children3,4,5,12). Obviously, after cerebrovascular event, neurocognitive function is impaired; however, even before the irreversible event, the chronically insufficient cerebral blood flow status can impact the patient's neurocognitive function1). Compared to pediatric patients, the neurocognitive impairment of adult patients diagnosed with MMD is underestimated and not thoroughly evaluated; thus, it is infrequently reported and, to date, has not been well described20). Recent reports have documented cognitive impairment in some adults with MMD11). In addition, there is some preliminary evidence that cerebral revascularization may slow or arrest progressive cognitive decline, or even improve cognition, in this disease10). However, these studies do not clearly define the effects of stroke or its sequelae. There were reports about neurocognitive profile of pediatric MMD patients pre- and post-revascularization surgery; however, to the best of our knowledge there are no analytic reports of cognitive dysfunction in adult MMD patients pre- and post-revascularization surgery14). We attempted to determine the neurocognitive profile and cognitive impairment of adult MMD patients and whether superficial temporal artery-middle cerebral artery (STA-MCA) bypass surgery can improve cognitive function manifesting as hemodynamic impairment without stroke.
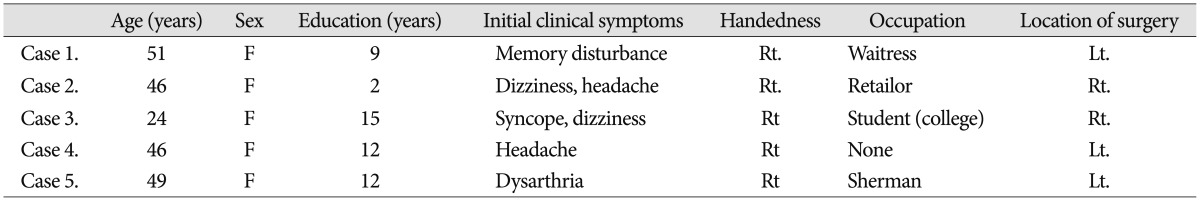
From September 2010 through November 2012, we identified 12 adult patients with angiographic confirmation of MMD; we designed a prospective study to compare and analyze cognitive function in patients with hemodynamic impairment before and after STA-MCA anastomosis surgery. Five patients fulfilled the study criteria described below; all five were females with a median age of 43.2 (range, 24-51 years). Initial clinical symptoms leading to the neurocognitive evaluation included two or more of following symptoms : dizziness, headache, syncope, memory disturbance, and/or dysarthria. The mean educational level of these patients was 10 years. A summary of patient characteristics is presented in Table 1.
All bypass surgeries were performed by one neurosurgeon. For preoperative evaluation, the patients underwent computed tomography 3D angiography (CTA), trans-femoral cerebral angiography (TFCA), brain perfusion single-photon emission computed tomography (SPECT) with and without acetazolamide injection, and magnetic resonance image (MRI) of the brain, which included following sequences : T1, T2, gradient echo (GRE), perfusion,and diffusion. Brain perfusion SPECT is a functional nuclear imaging technique performed to evaluate regional cerebral perfusion. We used SPECT Symbia dual e cam (Siemens Medical Solutions USA, Inc., Hoffmann Estates, IL, USA). The resting image is taken after injection of 15-30 mCi of 99mTc-hexamethylpropyleneamine oxime (HMPAO). The dynamic SPECT image is taken 10-20 minutes after intravenous injection of 1 g acetazolamide, which increases local pCO2 and causes arteriolar dilation; thus, allowing for assessment of cerebrovascular reserve. MRI perfusion and brain perfusion SPECT with and without acetazolamide were used to evaluate hemodynamic status. MR diffusion revealed the previous ischemic injury; this finding was used to eliminate the candidate who had or could have cognitive impairment due to stroke. The static image studies were all confirmed and interpreted by one neuroradiologist and five neurosurgeons. Among the 12 patients, five candidates satisfied all of the following criteria : 1) no evidence of cerebral infarction; 2) no evidence of cerebral hemorrhage; 3) no evidence of other brain lesions such as malignancy; 4) agreed to undergo pre- and post-operative evaluation including imaging studies and a neurocognitive evaluation. Seven were excluded for following reasons : five patients had evidence of a previous ischemic attack on MRI and two patients refused to participate in a postoperative follow-up evaluation.
The Seoul Neuropsychological Screening Battery (SNSB) is a comprehensive neurocognitive test that evaluates the following domains : attention and working memory (Digit Span Forward and Backward Test), frontal function tests (motor impersistence test, contrasting program, go/no-go test, alternating square and triangle test, Luria loop test, Stroop test, and word fluency test), verbal memory test (Korean version of the Hopkins Verbal Learning Test), visuospatial function and visual memory test (Rey Osterrieteh complex figure tests), and language tests (comprehension item, fluency item, repetition, reading, writing, and the Korean version of the Boston Naming Test). The test score results of each patient were compared with the standard scores of normal healthy subjects who were matched with respect to age, sex, and level of education. The Korean Mini-Mental State Examination (K-MMSE) was also applied9). The SNSB was used to compare the cognitive function state of the patients before and after the surgery; it was reviewed and interpreted by one examiner. Prior to surgery, all patients were assessed with the SNSB to evaluate their cognitive impairment due to chronic hypoperfusion; it was repeated within three months after the bypass surgery to evaluate any neurocognitive differences. The following categories were evaluated in all patients : general mental status, attention, confrontational naming, visuospatial function, verbal memory, visual memory, generative naming, and inhibitory control. From these domains, we specifically divided the memory domain into immediate recall and delayed recall. The standard paradigm to evaluate memory function involves the recall period starting immediately after reciting a list of three to four items; this can be referred to as immediate free recall (IFR). In delayed free recall (DFR), a 10-20 minute distraction period is interpolated between the first reminder from the examiner. For these measurements of SNSB, age-adjusted, education-adjusted z-scores were obtained. We determined cognitive impairment by the following criteria : mild dysfunction if the z-score was 1 to 2 SDs below the normative mean; moderate dysfunction if the score was 2 to 3 SDs below the normative mean; and severe if the score was ≥3 SDs below the normative mean. Each domain provided raw data, which was converted to z-scores, based on published normative means9,10,11).
Data from preoperative and postoperative cognitive function tests were compared and analyzed. Statistical evaluation was performed using the Wilcoxon signed rank test. The level of significance was set at p<0.05. Statistical analyses were performed using the SPSS Statistics 20 (IBM Corp., Armonk, NY, USA).
Data from five patients were analyzed in the present study. In all five cases, TFCA was performed prior to surgery to conform the presence of an occlusive lesion (Fig. 1A). The unilateral STA-MCA bypass surgery was performed successfully without complications (Fig. 1B, C). A preoperative MRI diffusion study found no evidence of infarction (Fig. 1D). Preoperative perfusion MRI showed MTT and TTP prolongation (Fig. 1E, F). Brain perfusion SPECT with and without acetazolamide confirmed decreased reserve capacity in the right frontal lesion (Fig. 1G, H). The postoperative TFCA showed adequate patency of the bypass; hemodynamic evaluation of the surgical sites via MRI perfusion and brain perfusion SPECT imaging with and without acetazolamide on postoperative day 7 showed improvement (Fig. 1I-L). The patients underwent a follow-up SNSB test within three 3 months of the STA-MCA bypass surgery. Data from pre- and post-operative cognitive function tests were compared and analyzed (Fig. 2, 3).
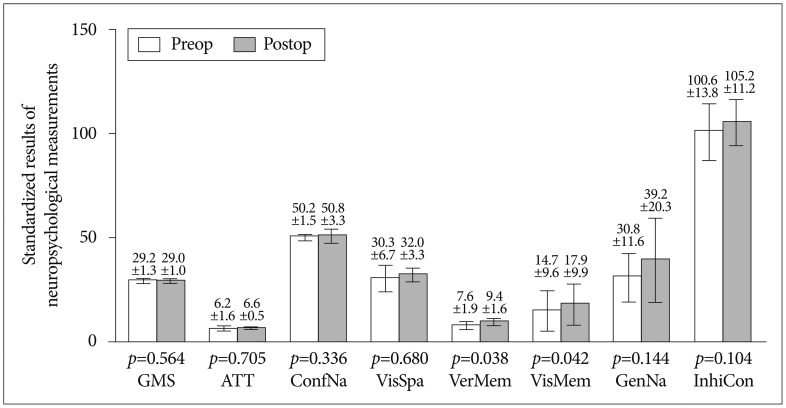
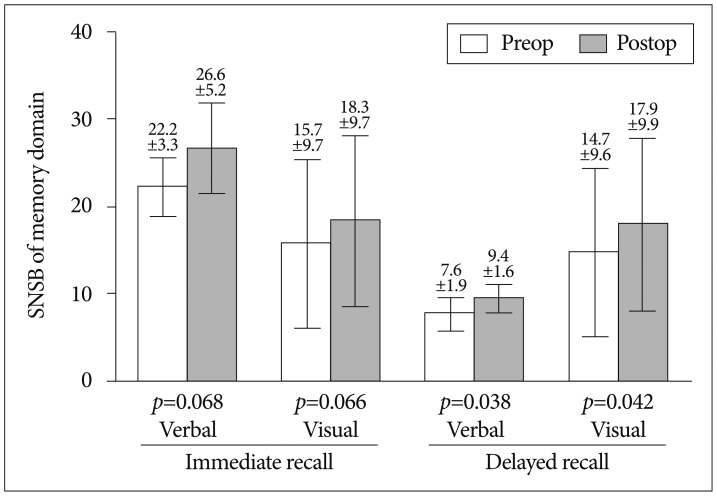
Pre- and post-operative MR perfusion and brain perfusion SPECT showed postoperative improvement of hemodynamic status. The perfusion MRI and SPECT images were compared (Fig. 1E-L). The postoperative MRI perfusion showed improvement in the MCA region where the STA is connected to the 4th segment of the MCA branch. In addition, the SPECT image showed improvement in reserve capacity. The results of the MRI perfusion and SPECT images correlated well and documented the successful surgery result with improved progression of hemodynamic status. Comparison of pre- and post-operative cognitive function revealed SNSB improvement. Fig. 2 shows the comparison of preoperative and postoperative z-score mean standardized results of neuropsychological measurements and the differences of the two results in eight domains. In general all patient results improved postoperatively except general mental status. However, these findings only attained statistical significance in the memory domain. Visuospatial function, inhibitory control, and memory are frequently impaired domains. Few patients had severe dysfunction (≥3 SDs below the normative mean) in specific domain. However, impairment of visuospatial function and inhibitory control domain were patient-specific rather than general; thus, the p-values of these domains were not statistically significant. For example, one patient scored -11.46 on the preoperative evaluation; it improved to -2.08 postoperatively, which represented an approximately 9 SD improvement in the visuospatial domain. The memory domain was the most improved domain following STA-MCA bypass, specifically in delayed recall. The visual memory domain improved in four of five patients. The memory domain was specifically analyzed in Fig. 3. Although both immediate and delayed recall improved, the improvement of delayed recall was statistically significant (p-values=0.038 for verbal memory and 0.042 for visual memory) together with the improvement of subjective symptoms.
MMD is a rare cerebrovascular disorder characterized by progressive stenosis and occlusion of the internal carotid and the anterior and middle cerebral arteries11). MMD is diagnosed via confirming the cerebrovascular occlusion of the distal internal carotid artery (ICA), proximal anterior cerebral artery (ACA) and/or the MCA via CTA, or TFCA13). MRI perfusion or brain perfusion SPECT with and without acetazolamide is used to evaluate the hemodynamic status of the brain due to chronic hypoperfusion from diminished vascularity15). Usually, MMD is diagnosed after a cerebral stroke in adults and a transient ischemic attack in children3,4,5,12). The need for revascularization surgery has been extensively researched5,16,20).
In children with MMD, diminished cerebral perfusion in MMD may lead to regional dysfunction in the absence of overt infarction8). Among pediatric patients, intellectual ability is an ongoing focus of research. Thus, there are many reports assessing intellectual ability. Furthermore, cognitive impairments are not infrequent in children with MMD, despite normal general intellectual function,7) William et al.21) reported that children with MMD demonstrated lower intelligence and poorer executive function than test standardization samples. It is a recent concept that in adult MMD patients, chronic hypoperfusion can lead to cognitive impairment. Jefferson et al.8) emphasized the importance of cognitive function evaluation for chronic cerebrovascular disorders including MMD. Karzmark et al.10) documented that MMD in adults can impair cognition, particularly in the domain of executive function. Calviere et al.1) reported a high rate of cognitive impairment in six of 10 patients that significantly affected executive function.
Frequently, the cognitive impairment in adult patients is underestimated and neglected. Mogensen et al.15) reported decreased executive function in an adult population with MMD; it was most strongly associated with secondary damage to the brain parenchyma, manifested by white matter disease or cortical stroke. Diagnosis of MMD in adult patients following stroke, either ischemic or hemorrhagic, obscures the influence of chronic hypoperfusion; consequently, cognitive dysfunctions are frequently not assessed or misinterpreted. Because the cognitive impairment slowly progresses over time17,18) and many patients assume that the symptoms are due to the aging process rather than chronic hypoperfusion. There are some reports, both in children14,20) and in adults1,20) that note that cerebral revascularization may slow or arrest progression and perhaps even improve cognition; however, these studies do not clearly define the effects of stroke.10) Lee et al.14) analyzed improvement in the level of intelligence after indirect bypass surgery in pediatric MMD patients; they found that the long-term outcome of cognitive function was poorer in the nonsurgical group. These reports were limited to pediatric patients. In the current study, the samples were adult MMD patients without a history of a cerebrovascular event, and all patients enrolled in this study were evaluated for an undiagnosed stroke event and present hemodynamic status. We analyzed the cognitive improvement comparing pre- and post-revascularization surgery in stroke-free adults.
The SNSB is one of the most commonly used neuropsychological tests in Korea for assessing cognitive function; the normal range is based on the analysis of 447 healthy subjects. The standard test indices are age, education, and sex (adjusted to interpretation)9). Comparing the pre- and post-operative cognitive function test, the results of postoperative cognitive function test showed substantial improvement in the memory domain together with the recovery of the patients' subjective symptoms. The results of the postoperative cognitive function test showed improvement, compared to preoperative status. The neurocognitive impairment differs between adult and pediatric populations. Intelligence is the most affected cognitive parameter in children, whereas executive function is the most impaired parameter in adults20). The pediatric samples with MMD had an almost normal, age-appropriate level of intelligence and neurocognitive function before surgery and their cognitive function appeared to be maintained postoperatively14). For adult MMD patients, the focus of the cognitive impairment should be on executive function and memory domains. The preoperative results of impaired cognition domains were similar to those of previous studies. Mental efficiency, executive function, and expressive language abilities were found to be the domains most affected in previous reports1,8,10,15,20). Memory and basic sensory capacities were reported to be relatively intact in other studies, which differed from the present study. Weinberg et al.20) concluded that of all cognitive parameters tested, memory showed the lowest rate of impairment; therefore, the medial temporal function is relatively spared in patients with MMD. In contrast, our study showed that memory and visuospatial functions were the most impaired, followed by executive function. In all five patients, statistically significant improvement was found in the memory domain, specifically delayed recall memory. Since MMD is a disease primarily affecting the distal ICA and proximal middle and anterior cerebral artery, usually the ACA and MCA regions and their watershed areas are affected2,6,19). The impairment of frontal perfusion in MMD can be explained by the distribution of arterial occlusive lesions, usually limited to the ICAs, and sparing the posterior circulation, and the development of a pial collateral supply from the posterior cerebral arteries to the middle and anterior cerebral arteries1). As a result, the executive, memory domains could be impacted by chronic hypoperfusion; thus, they would benefit the most from revascularization.
Our study has several limitations. First, there was a limitation in patient selection. Because all the patients were selected from symptomatic individuals who sought medical care, there was selection bias. Enrolled samples were biased toward patients with the greatest neurocognitive impairment, with variable clinical symptoms such as headache, dizziness, or even transient ischemic attacks. Second, because MMD is a rare disease it does not comprise an adequate sample size to detect potential differences in race, gender or institution. Third, our study is limited to patients who underwent revascularization surgery. Fourth, the difference of neurocognitive impairment between the non-surgical group and the surgical group were not compared. Therefore, we do not have complete understanding of the progression of cognitive impairment under conservative management. Fifth, SNSB can evaluate all cognitive domains; however, the battery does not provide a global cognitive function (GCF) score. The standard index of age range was 55 to 80 years with a mean age of 67.63 years (female : 67.51; male 67.79)9). The mean age of our patients was 43.2 years, which was younger than the standard index of the exam; therefore, there is uncertainty in regard to the results regarding the age factor. The results may appear milder than the actual degree of cognitive impairment. Also the duration to complete the exam is approximately 90-120 minutes. For some patients, it is a long enough time for inattention to occur, which would compromise the results. Further studies that comprise a larger patient population with pre- and post-operation neurocognitive evaluation are essential for the conduction of a multivariable analysis.
In our preliminary study, we found that a large proportion of patients with adult MMD demonstrated disruption of neurocognition. Cognitive impairment could ensue after a focal neurologic deficit; this could be followed by a hemorrhagic event or infarction. However, we also suggest the possibility of chronic hypoperfusion as the primary cause of the cognitive impairment in MMD. The result of the SNSB comparing preoperative and postoperative STA-MCA bypass surgery showed remarkable improvements in the memory domain. Although further study is needed, the importance of performing a neurocognitive evaluation should be emphasized. This would more comprehensively assess the full clinical impact of MMD; furthermore, cognitive dysfunction may be an indicator for earlier intervention with perfusion procedures that would benefit cognitive function.
References
1. Calviere L, Catalaa I, Marlats F, Viguier A, Bonneville F, Cognard C, et al. Correlation between cognitive impairment and cerebral hemodynamic disturbances on perfusion magnetic resonance imaging in European adults with moyamoya disease. Clinical article. J Neurosurg. 2010; 113:753–759. PMID: 20469988.

2. Chang KH, Yi JG, Han MH, Kim IO. MR imaging findings of moyamoya disease. J Korean Med Sci. 1990; 5:85–90. PMID: 2278666.

3. Fujii K, Ikezaki K, Irikura K, Miyasaka Y, Fukui M. The efficacy of bypass surgery for the patients with hemorrhagic moyamoya disease. Clin Neurol Neurosurg. 1997; 99(Suppl 2):S194–S195. PMID: 9409436.

4. Fukui M, Kono S, Sueishi K, Ikezaki K. Moyamoya disease. Neuropathology. 2000; 20(Suppl):S61–S64. PMID: 11037190.

5. Guzman R, Lee M, Achrol A, Bell-Stephens T, Kelly M, Do HM, et al. Clinical outcome after 450 revascularization procedures for moyamoya disease. Clinical article. J Neurosurg. 2009; 111:927–935. PMID: 19463046.

6. Hayashi T, Shirane R, Fujimura M, Tominaga T. Postoperative neurological deterioration in pediatric moyamoya disease : watershed shift and hyperperfusion. J Neurosurg Pediatr. 2010; 6:73–81. PMID: 20593991.

7. Hsu YH, Kuo MF, Hua MS, Yang CC. Selective neuropsychological impairments and related clinical factors in children with moyamoya disease of the transient ischemic attack type. Childs Nerv Syst. 2014; 30:441–447. PMID: 24005800.

8. Jefferson AL, Glosser G, Detre JA, Sinson G, Liebeskind DS. Neuropsychological and perfusion MR imaging correlates of revascularization in a case of moyamoya syndrome. AJNR Am J Neuroradiol. 2006; 27:98–100. PMID: 16418365.
9. Kang Y, Na DL. Seoul Neuropsychological Screening Battery (SNSB). Seoul: Human Brain Research & Consulting Co.;2003.
10. Karzmark P, Zeifert PD, Bell-Stephens TE, Steinberg GK, Dorfman LJ. Neurocognitive impairment in adults with moyamoya disease without stroke. Neurosurgery. 2012; 70:634–638. PMID: 21849919.

11. Karzmark P, Zeifert PD, Tan S, Dorfman LJ, Bell-Stephens TE, Steinberg GK. Effect of moyamoya disease on neuropsychological functioning in adults. Neurosurgery. 2008; 62:1048–1051. discussion 1051-1052. PMID: 18580802.

12. Kuroda S, Ishikawa T, Houkin K, Nanba R, Hokari M, Iwasaki Y. Incidence and clinical features of disease progression in adult moyamoya disease. Stroke. 2005; 36:2148–2153. PMID: 16179571.

13. Kwag HJ, Jeong DW, Lee SH, Kim DH, Kim J. Intracranial hemodynamic changes during adult moyamoya disease progression. J Clin Neurol. 2008; 4:67–74. PMID: 19513306.

14. Lee JY, Phi JH, Wang KC, Cho BK, Shin MS, Kim SK. Neurocognitive profiles of children with moyamoya disease before and after surgical intervention. Cerebrovasc Dis. 2011; 31:230–237. PMID: 21178347.

15. Mogensen MA, Karzmark P, Zeifert PD, Rosenberg J, Marks M, Steinberg GK, et al. Neuroradiologic correlates of cognitive impairment in adult Moyamoya disease. AJNR Am J Neuroradiol. 2012; 33:721–725. PMID: 22173751.

16. Nakagawa Y, Abe H, Sawamura Y, Kamiyama H, Gotoh S, Kashiwaba T. Revascularization surgery for moyamoya disease. Neurol Res. 1988; 10:32–39. PMID: 2899853.

17. Peerless SJ. Risk factors of moyamoya disease in Canada and the USA. Clin Neurol Neurosurg. 1997; 99(Suppl 2):S45–S48. PMID: 9409404.

18. Scott RM, Smith ER. Moyamoya disease and moyamoya syndrome. N Engl J Med. 2009; 360:1226–1237. PMID: 19297575.

19. Smith JL. Understanding and treating moyamoya disease in children. Neurosurg Focus. 2009; 26:E4. PMID: 19335128.

20. Weinberg DG, Rahme RJ, Aoun SG, Batjer HH, Bendok BR. Moyamoya disease : functional and neurocognitive outcomes in the pediatric and adult populations. Neurosurg Focus. 2011; 30:E21. PMID: 21631223.
21. Williams TS, Westmacott R, Dlamini N, Granite L, Dirks P, Askalan R, et al. Intellectual ability and executive function in pediatric moyamoya vasculopathy. Dev Med Child Neurol. 2012; 54:30–37. PMID: 22117564.

Fig. 1
A 46-years-old woman with dizziness underwent right STA-MCA anastomosis surgery. A : Preoperative TFCA image shows bilateral distal ICA occlusion with development of collateral vessels. White arrows indicate moyamoya vessels in both ICAs. B and C : STA-MCA anastomosis was performed in the right frontoparietal area. Postoperative TFCA was performed and STA-MCA anastomosis flow was present. D : There was no evidence of acute stoke with a preoperative diffusion imaging study. E and F : Preoperative MRI perfusion shows prolongation of MTT (E) and TTP (F) in both hemispheres, especially in the right frontal area. G (without acetazolamide) and H (with acetazolamide) : Brain perfusion SPECT performed to evaluate preoperative reserve capacity of the patient; it revealed a suspicion of impaired reserve capacity in the right frontal lobe. I and J : Post-operation image study shows improvement of MTT (I) and TTP (J) prolongation and hemodynamic status in the right frontal lobe. White arrow shows the perfusion improvement following surgery. K (without acetazolamide) and L (with acetazolamide) : Brain perfusion SPECT performed on postoperative day 7 shows improvement in reserve capacity when the preoperative acetazolamide stress test is compared to the postoperative acetazolamide stress test. STA-MCA : superficial temporal artery-middle cerebral artery, TFCA : trans-femoral cerebral angiography, ICA : internal carotid artery, MTT : mean transit time, TTP : time to peak, SPECT : single photon emission computerized tomography.
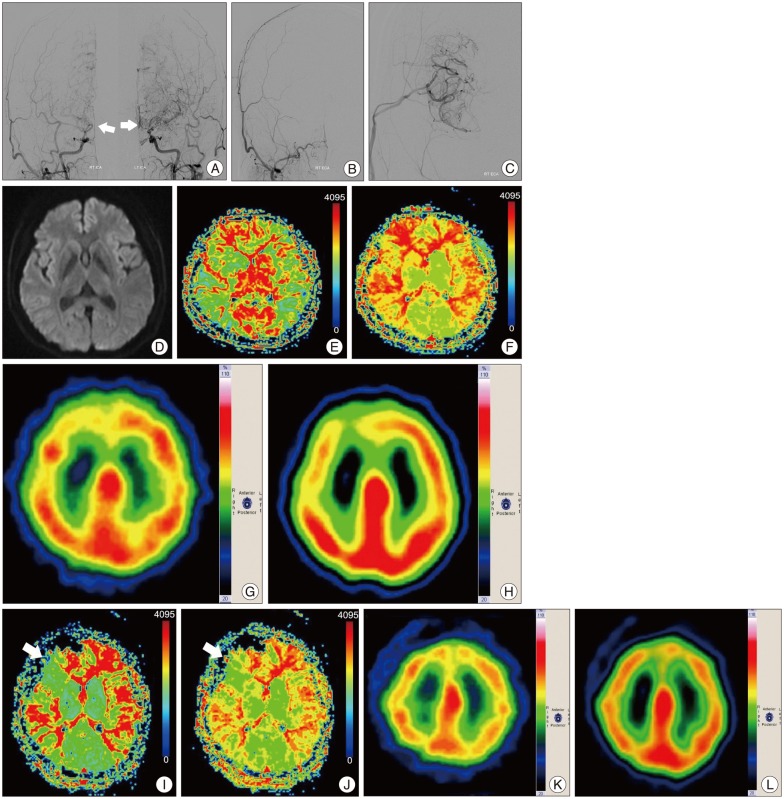
Fig. 2
Comparison of preoperative and postoperative neurocognitive function tests in eight domains. Generally, postoperative function (in black) shows improvement compared to preoperative results except general mental status. Each error bar denotes standard deviation. GMS : general mental state, ATT : attention, ConfNa : confrontational naming, VisSpa : visuospatial function, VerMem : verbal memory, VisMem : visual memory, GenNA : generative naming, InhiCon : inhibitory control. Verbal memory and visual memory domain showed significant improvement (p-values=0.038 and p-values=0.042).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download