Abstract
Objective
At present, gold-standard technique of cervical cord decompression is surgical decompression and fusion. But, many complications related cervical fusion have been reported. We adopted an extended anterior cervical foraminotomy (EACF) technique to decompress the anterolateral portion of cervical cord and report clinical results and effectiveness of this procedure.
Methods
Fifty-three patients were operated consecutively using EACF from 2008 to 2013. All of them were operated by a single surgeon via the unilateral approach. Twenty-two patients who exhibited radicular and/or myelopathic symptoms were enrolled in this study. All of them showed cervical cord compression in their preoperative magnetic resonance scan images.
Results
In surgical outcomes, 14 patients (64%) were classified as excellent and six (27%), as good. The mean difference of cervical cord anterior-posterior diameter after surgery was 0.92 mm (p<0.01) and transverse area was 9.77 mm2 (p<0.01). The dynamic radiological study showed that the average post-operative translation (retrolisthesis) was 0.36 mm and the disc height loss at the operated level was 0.81 mm. The change in the Cobb angle decreased to 3.46, and showed slight kyphosis. The average vertebral body resection rate was 11.47%. No procedure-related complications occurred. Only one patient who had two-level decompression needed anterior fusion at one level as a secondary surgery due to postoperative instability.
Spondylotic lesions that cause cervical cord compression require surgical decompression either through anterior cervical discectomy/corpectomy with fusion or laminectomy/laminoplasty when they result in progressive neurological deficits2,3,13,20). These procedures have been regarded as a gold-standard technique for decompression and stabilization. However, fusion-related problems such as loss of the cervical range of motion, non-union, instrumentation failure, and graft extrusion are still the subject of debates7,20).
The Anterior cervical microforaminotomy (ACMF) technique involves not only the direct removal of the compressive abnormality but also the preservation of the motion segments without bone fusion or post-operative immobilization9,11,15).
ACMF was originally developed for cervical radiculopathy. But, it naturally advanced to spinal cord decompression with extended technique (extended anterior cervical foraminotomy, EACF). Although many previous studies showed favorable clinical and radiological results, it has been used only for nerve root decompression. Here, we report the clinical results and effectiveness of this procedure for cervical cord decompression.
Between September 2008 and January 2013, we performed 53 EACF procedures. All our patients were operated on by a single surgeon via the unilateral approach. Twenty-two of the patients who showed cervical cord compression on their pre-operative magnetic resonance scan images with radicular and/or myelopathic symptoms were enrolled in this study. The preoperative instability on cervical dynamic X-ray and already pre-existing narrowing of cervical spinal canal (that is, developmental stenosis) excluded in this study.
The detailed surgical technique of ACMF for cord compression has been reported by Jho8). A brief summary of our procedure from skin incision to exposure of the anterior column of the cervical spine is similar to other anterior approach to the cervical spine. An anterior cervical discectomy retractor system is applied to expose the ipsilateral longus colli muscle rather than the midline anterior disc surface. An operating microscope is essential for next stage. The lateral portion of the longus colli muscle is excised to expose the medial parts of the transverse processes of the upper and lower vertebrae. Once the medial portion of the transverse processes of the upper and lower vertebrae is identified, the ipsilateral uncovertebral joint between them can be seen; however, in advanced spondylosis, anatomical landmarks of the uncovertebral joint and transverse processes cannot be delineated. The uncovertebral joint is removed between the transverse processes using a high-speed microsurgical drill attached to an angled hand piece. To prevent injury to the vertebral artery (VA), a thin layer of cortical bone is left attached to the ligamentous tissue covering the medial portion of this artery. Drilling continues down to the posterior longitudinal ligament. As drilling advances posteriorly, the direction of the drill is gently inclined medially. When the posterior longitudinal ligament is exposed, a piece of thin cortical bone is left attached laterally to the periosteal and ligamentous tissue covering the VA.
This lateral remnant of the uncinate process is dissected from the ligamentous tissue and fractured at the base of the uncinate process. It is further dissected from the surrounding soft tissue and removed, which enables identification of the VA by its pulsation between the transverse processes of the vertebrae. It is necessary to proceed cautiously with drilling at the base of the uncinate process because the nerve root lies just adjacent to it. After the uncinate process becomes loosened at its base, it is safer to remove the thin layer of remaining bone of the uncinate process by fracturing it rather than by continued drilling. After remained piece of the uncinate process is removed, extended foraminotomy procedure started. The posterior osteophytes, herniated part of disc, some parts of the upper and lower endplates are removed. The procedure crossed over the midline diagonally toward the opposite margin of the spinal dura mater.
The posterior longitudinal ligament is incised and resected to achieve adequate decompression of the ipsilateral nerve root and spinal cord. Sometimes the beginning of the contralateral nerve root is identified for adequate decompression of the spinal canal in the transverse axis. Multiple anterior foraminotomies are performed as needed. Using the holes of anterior foraminotomies, the spinal cord canal is enlarged in the longitudinal axis by removing the posterior portion of the vertebral bodies with Kerrison rongeurs and a long-armed up-biting curette.
All the patients took pre-operative plain radiography of cervical spine, computed tomography (CT), magnetic resonance imaging (MRI), and dynamic plain radiography of cervical spine in six weeks after their operation. Analyses of the pre- and post-operative dynamic plain radiographs were done to evaluate postoperative instability.
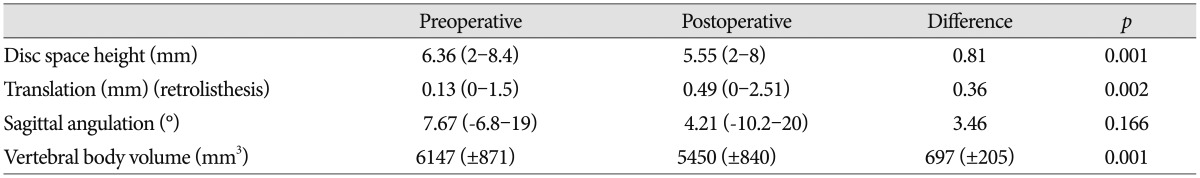
We measured five radiological data for evaluate to presence of postoperative instability and effectiveness of cervical cord decompression : the disc space height (DSH), sagittal plane displacement (translation), Cobb angle, vertebral body resection rate and degree of cervical cord decompression.
The disc space height was measured from the mid-point of the upper vertebral body to the mid-point of the lower vertebral body on the sagittal plane. To allow different magnification factors on different films, the measurements were compared with the height of the third cervical vertebrae (C3)18).
The sagittal plane displacement was measured on the cervical flexion-extension radiographs as the linear distance in millimeter scale from the posterior-inferior corner of the superior vertebra to the posterior-superior corner of the inferior vertebra body. A distance greater than 3.5 mm defined instability19).
The sagittal plane alignment was defined on the lateral neutral radiographs in relation to the line that joined the postero-inferior edge of C2 to the postero-inferior edge of C7. When all the intervening vertebral bodies were found anterior to this line, the alignment was defined as a lordotic curvature.
The sagittal angulation, which is the angle between a line on the superior end plate of the vertebral body above and a line on the inferior border of the body below the levels operated 1), was measured on the neutral lateral view at the operated level, using Cobb's method.
The vertebral body resection rate was measured on pre- and post-cervical 3-dimensional CT scan. All scans were performed on a 64-slice multi-detector computed tomography scanner (TOSHIBA Aquilion, Tokyo, Japan). The scanning parameters were as follows : voltage of 120 kV, current 150 mAs, field-of-view 147 mm and slice thickness of 3 mm (Fig. 1).
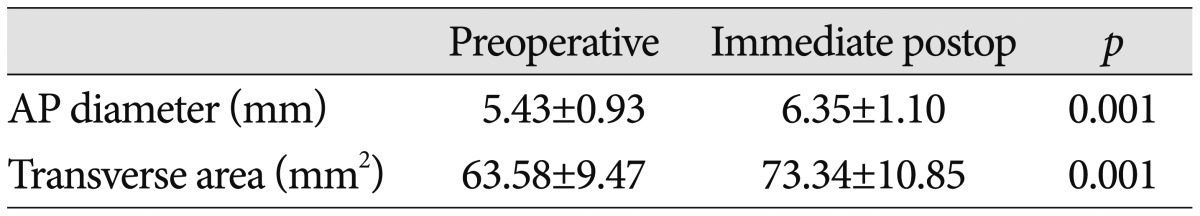
The degree of cervical cord decompression was measured antero-posterior diameter and transverse area of the spinal cord at the site of maximal compression. The data from preoperative and postoperative T2-weighted axial MRI analyzed using Aquarius iNutrition Edition ver4.4.6. (TeraRecon, Foster City, CA, USA) (Fig. 1).
Signal changes in the spinal cord on the T2 weighted images were also noted.
The post-operative pain improvement was measured using the visual analogue score (VAS). The clinical outcome was graded as "excellent" when the patient showed complete resolution of all symptoms, "good" when the patient experienced relief of radiculopathy but still experienced occasional minimal/mild residual non-radicular discomfort, "fair" when the patient showed mild residual symptoms of radiculopathy with or without mild/moderated residual non-radicular discomfort, and "poor" when the patient continued to show significant radicular symptoms with or without non-radicular discomfort.
The statistical analysis was performed using PASW statistics 18.0 (SPSS Inc., Chicago, IL, USA). We were analyzed to the degree of cervical cord decompression, disc space height loss, translation, Cobb angle and vertebral body resection rate using paired t-test. A p value of 0.05 was considered significant.
A 52-year-old woman was referred to our hospital with a 6-month history of posterior neck pain, right shoulder pain and progressive myelopathy that both upper and lower extremity numbness (right>left). On neurologic examination, she had Grade 4-/5 weakness of her right elbow flexion and extension. Her biceps and triceps reflexes were normal range. Spurling's sign and Lhermitte's sign were present bilaterally. CT and MRI scans demonstrated bulging disc C4-5, C5-6 with focal ossification posterior longitudinal ligament and showed cervical cord compression.
The patient underwent spinal cord decompression via right-sided microsurgical anterior foraminotomy holes at C4-5, C5-6. Postoperative CT and MR imaging confirmed generous decompression of their spinal canal (Fig. 2). She stayed 9 days in the hospital postoperatively. At discharge, her radiculomyelopathy had improved and improvement Rt. Biceps and triceps weakness. On follow up examination after 6 weeks, she showed no instability on a flexion-extension dynamic roentgenogram of the cervical spine (Fig. 3).
The average follow-up duration was 30.36 months (ranged 9-57 months). The age of the patients ranged 29-75 years (median 55 years). Seventeen of the patients were male and five were female. Duration of the occurrence of the symptoms was four weeks to 10 years (median 12 months). In addition to the symptoms and signs of myelopathy, 22 patients (100%) had radicular symptoms and motor weakness, 18 patients (82%) had a sensory deficit, four patients (18%) had gait disturbance, and one patient (5%) had a bladder symptom (Table 1).
Single-level operations were performed on five patients, and two-level operations were done on 17 patients. The operative levels were C4-5 for 10 patients, C5-6 for 20 patients and C6-7 for nine patients.
Fourteen patients (64%) showed excellent results; six patients (27%), good results; and one patient (4%), a fair result. A total of 91% of the patients had an excellent or good outcome. However, one patient (5%) had a poor result, and he was re-operated on due to post-operative instability (Table 2).
The mean pre-operative VAS score was 7.5 (±1.01), the post-operative VAS score was 1.8 (±0.85), and the VAS score difference was 5.7.
The mean preoperative disc space height (DSH) was 6.36 (2-8.4) mm, and the post-operative DSH was 5.5 (2-8) mm. The mean DSH loss was 0.81 mm (p<0.001). The mean pre-operative translation (retrolisthesis) was 0.13 (0-1.5) mm, and the post-operative translation was 0.49 (0-2.51) mm. The mean retrolisthesis was 0.36 mm (p<0.002). The mean pre-operative Cobb angle in the neutral position was 7.67° (-6.8°-19°), and the mean post-operative Cobb angle was 4.21° (-10.2°-20°). The change in the Cobb angle decreased by 3.46 and showed slight kyphosis (p<0.166). The average vertebral body resection rate was 11.47% (8.10-16.14%, p<0.001) (Table 3).
The mean preoperative AP diameter at the site of maximal com-pression improved from 5.43±0.93 mm preoperatively to 6.35 ± 1.10 mm, postoperatively (p<0.001). And the mean preoperative transverse area at the site of maximal compression improved from 63.58±9.47 mm2 preoperatively to 73.34±10.85 mm2, postoperatively (p<0.001) (Table 4).
Cervical cord compressing lesions may eventually develop myelopathic symptoms. But some patients presented only radicular symptoms as current clinical manifestations. Even in patients who had radicular symptoms, we frequently observed spinal cord compression in MRI and CT scan. In the literature, seventy-five percent of patients with myelopathy deteriorate in a stepwise fashion, 20% deteriorate slowly and steadily, and 5% have a rapid onset of symptoms with a stable plateau of dysfunction5). Most patients require surgical decompression when their condition results in progressive neurological deficit and functional declination. Traditionally, surgical decompression has been performed to arrest neurological deterioration and prevent further disability. However, recent studies have shown improved clinical outcomes.
At present, surgical treatment of cervical myelopathy can be broadly divided into anterior and posterior approaches. The anterior approaches include anterior cervical discectomy and corpectomy, while the posterior approaches involve laminectomy with or without fusion, and laminoplasty12,13,17,21). The choice between an anterior or posterior approach depends on the location, extent and type of the compressive pathology, the curvature of the spine, and the presence of instability. Like this, various techniques can be used presently, but the main goal of surgical intervention is spinal cord decompression. Optimal treatment of CSM is still controversial, and fusion-related complications such as loss of the range of motion, non-union, instrumentation failure and graft extrusion, and the adjacent-segment syndrome were exist20,22). In our study, although all patients are not enough to criteria of cervical spondylotic myelopathy, main pathologic finding is cervical cord compression with degenerative change such as stenosis, OPLL. Through this study, we evaluate the effectiveness of EACF technique to CSM patients.
We propose EACF as an alternative minimally invasive non-fusion technique for cervical cord decompression. The ECAF technique is originally based on ACMF; actually it's one of technical variation. Most surgeons have accepted the ACMF as a surgical procedure for cervical nerve root decompression. So, we suggest changing the name of this technical variation into extended technique in descriptive terminology. It seems to be more practical and rational to understand the procedure. Some authors reported anterolateral technique to decompress the cervical cord, but these procedures were targeted for vertebral body not to the foramen where nerve root trespassing2,3). In the extended ACF, decompression started form the exit of the intervertebral foramen and proceeded into more anterolateral portion of the spinal canal. Intervertebral foramen offers a natural anatomical corridor to lead to the spinal canal. Along with extended resection from the pathologic part of the foramen to the anterolateral portion of the cord, a funnel shaped widened area can be obtained. This space contained a decompressed nerve root and spinal cord without a large area of bony resection. So this terminology, extended anterior cervical foraminotomy technique, is more reasonable to describe this procedure for cord decompression.
ACMF was developed by Jho et al.10) under the concept of "functional spine surgery," which directly eliminates the compressive pathological factor while preserving the functional anatomic features. This minimally invasive surgical procedure is widely used in cervical radiculopathy and has shown favorable outcomes in many studies. This technique was also used in cervical myelopathy in 2002 and had good surgical results. In his study, 40 patients were treated with ACMF. Eighty percent of them showed good to excellent surgical results, with minimal morbidities, six weeks after their operation, and 90% of them showed the same results at the long-term follow-up. Spinal stability was well maintained in all the patients9). The clinical results of our study are comparable to the aforementioned study. A total of 91% patients had an excellent or good outcome and no procedure related complication occurred. But, his study was not assessed the radiologic outcomes. In this reasons, we measured five radiologic outcomes. And except the vertebral body resection rate and degree of cervical cord decompression, radiologic outcome was similar to other studies that were ACMF for radiculopathy patients11,14,15).
Therefore, our study results support the extended ACF for cervical cord decompression is safety and effectiveness.
The main advantages of this procedure are the quick patient recovery without the need for bone fusion, and the preservation of the motion segments. In generally, the anterior cervical discectomy and fusion (ACDF) procedure provides good access to the spinal canal, but not to the neural foramen. However, this procedure enables foraminal decompression through the same hole for patients with mixed myelopathy and radiculopathy symptoms. Although ACMF has many advantages, its complications must be considered.
ACMF has been associated with procedure-related complications and post-operative instability. The overall incidence of complications is known to be 7%10) to 22%,6) with Horner's syndrome (5.3%), hoarseness (4.8-5.9%), VA injury (1%), recurrence (1-17%) and discitis (1%) reported. In our study, no procedure related complications occurred. However, one patient had postoperative instability.
Some studies had postulated that uncovertebral joint resection with a degenerative disc leads to hypermobility, which results in a painful joint and post-operative instability. However, resection of the uncovertebral joint does not correctly describe anterior cervical foraminotomy. To maintain spinal stability and cervical spine motion, the ACMF technique was developed. It was developed by placing the foraminotomy hole more laterally and making it progressively smaller. Recently, Kim14) reported the surgical long-term outcome of ACMF for cervical degenerative disease. He reported that the disc space height decreased more when the ACMF diameter was more than 4.7 mm, and that the ideal range of the ACMF diameter may be from 2.6 mm to 4.7 mm.
Concerning the biomechanical effect, Chen et al.4) studied the relative biomechanical contributions of various structures in seven human cadaver C4-T1 specimens. The various destructive steps, which included discectomy, unilateral uncinate process removal, bilateral uncinate process removal, and posterior longitudinal ligament transection, were performed with biomechanical testing. It was reported that the preservation of much of the anterior structures, including the uncinated process, achieved better cervical stability when cervical fusion was not performed. Moreover, Kotani et al.16) emphasized the importance of maintaining the uncinated process. However, these biomechanical tests do not accurately represent the actual methods currently being used. Unlike with the actual anterior cervical microforaminotomy, these results were obtained after removing the uncinate process or much of the surrounding structures. Thus, these tests are inaccurate. Also, unlike previous assumptions that the disc space height will decrease and the retrolisthesis and sagittal rotation will increase and add to the instability due to the degeneration of the uncovertebral joint long after the surgery, there are cases in which the uncovertebral joint is fused as the foraminotomy hole is filled due to bone remodeling. This is similar to the reported bone reformation, a long time after posterior decompression, as in lumbar laminectomy and also in cases when the uncovertebral joint is retained without being fused but instead, filled up11).
There are many studies on the instability that follows after ACMF surgery in radiculopathy patients. Our study, however, focused on cord decompression. For this reason, the vertebral body resection rate in our study would be different from previous studies. Cord decompression requires more vertebral body resection. Therefore, Postoperative DSH loss and instability may occur frequently. We figured out the mean rate of vertebral body resection. The average vertebral body resection rate was 11.47%. The actual body resection rate was lower than we expected. But, one patient developed instability after surgery despite of mean vertebral body resection rate was 9%. The patient was young and had a soft disc, flexible neck without spondylotic change and a unilateral approach was used to resect both lesions. Conclusionally, the patient was not truly cervical spondylotic myelopathy and all these factors could be attributed to postoperative instability. Therefore, patients with these factors should consider cervical fusion rather than EACF.
Eventually, a dynamic stenosis may occur after decompression only surgery for cervical spondylotic myelopathy patients. But, our study did not show any symptoms related to restenosis during the follow up period. Concerning to this fact, our follow up periods seemed to be relatively short. In long-term follow up, some patients will develop restenosis and may need further surgery according to its pathological conditions. Nevertheless, we emphasized to preservation of motion segment as a primary advantage. And this procedure will contribute to decrease the risk of developing adjacent segment disease as compared with fusion surgery. Also, cervical spondylotic myelopathy is a degenerative disease that occurs mostly in older patients. Most of them exhibit spondylotic changes and will become ankylotic. Therefore, a better surgical strategy is to preserve the motion unit rather than the bone fusion.
This study had two limitations. First, it is a retrospective study with the subjects being mostly cervical cord decompression patients rather than symptomatic myelopathy only patients. As a result, the reliability of their clinical symptoms might have been low. Second, the follow-up intervals were variable and relatively short.
A decompression and fusion surgery has been considered as a standard surgical treatment for cervical cord compressing lesions. But, in our study, a decompression without fusion procedure was successfully performed using EACF technique. This procedure achieves adequate anterior decompression of the spinal cord with preservation of the functional motion, and avoids fusion-related complications. With our clinical and radiological results, authors conclude that EACF is a possible and applicable surgical option for the spondylotic patients who need cervical cord decompression.
In selecting patients, the pre-operative clinical status should be carefully considered to achieve an excellent outcome. Long-term follow-up and a further study in a larger series are needed.
References
1. Batzdorf U, Batzdorff A. Analysis of cervical spine curvature in patients with cervical spondylosis. Neurosurgery. 1988; 22:827–836. PMID: 3380271.

2. Chacko AG, Joseph M, Turel MK, Prabhu K, Daniel RT, Jacob KS. Multilevel oblique corpectomy for cervical spondylotic myelopathy preserves segmental motion. Eur Spine J. 2012; 21:1360–1367. PMID: 22234720.

3. Chacko AG, Turel MK, Sarkar S, Prabhu K, Daniel RT. Clinical and radiological outcomes in 153 patients undergoing oblique corpectomy for cervical spondylotic myelopathy. Br J Neurosurg. 2014; 28:49–55. PMID: 23859056.

4. Chen TY, Crawford NR, Sonntag VK, Dickman CA. Biomechanical effects of progressive anterior cervical decompression. Spine (Phila Pa 1976). 2001; 26:6–13. discussion 14. PMID: 11148638.

5. Clarke E, Robinson PK. Cervical myelopathy : a complication of cervical spondylosis. Brain. 1956; 79:483–510. PMID: 13364095.

6. Hacker RJ, Miller CG. Failed anterior cervical foraminotomy. J Neurosurg. 2003; 98(2 Suppl):126–130. PMID: 12650395.

7. Haden N, Latimer M, Seeley HM, Laing RJ. Loss of inter-vertebral disc height after anterior cervical discectomy. Br J Neurosurg. 2005; 19:469–474. PMID: 16574558.

8. Jho HD. Decompression via microsurgical anterior foraminotomy for cervical spondylotic myelopathy. Technical note. J Neurosurg. 1997; 86:297–302. PMID: 9010435.

9. Jho HD, Kim MH, Kim WK. Anterior cervical microforaminotomy for spondylotic cervical myelopathy : part 2. Neurosurgery. 2002; 51(5 Suppl):S54–S59. PMID: 12234430.
10. Jho HD, Kim WK, Kim MH. Anterior microforaminotomy for treatment of cervical radiculopathy : part 1--disc-preserving "functional cervical disc surgery". Neurosurgery. 2002; 51(5 Suppl):S46–S53. PMID: 12234429.
11. Jung SS, Chung JC, Park KS, Chung SY, Park MS, Ha HG. Long-term follow-up results of anterior cervical microforaminotomy. Korean J Spine. 2010; 7:66–72.
12. Kalsi-Ryan S, Karadimas SK, Fehlings MG. Cervical spondylotic myelopathy : the clinical phenomenon and the current pathobiology of an increasingly prevalent and devastating disorder. Neuroscientist. 2013; 19:409–421. PMID: 23204243.
13. Kim JG, Kim SW, Lee SM, Shin H. Surgical result of the combined anterior and posterior approach in treatment of cervical spondylotic myelopathy. J Korean Neurosurg Soc. 2006; 3:188–191.
14. Kim MH. Clinical and radiological long-term outcomes of anterior microforaminotomy for cervical degenerative disease. Spine (Phila Pa 1976). 2013; 38:1812–1819. PMID: 24150278.

15. Kim YG, Lee JS, Park MS, Ha HG. Midterm follow-up results of anterior cervical microforaminotomy. J Korean Neurosurg Soc. 2004; 35:250–255.
16. Kotani Y, McNulty PS, Abumi K, Cunningham BW, Kaneda K, McAfee PC. The role of anteromedial foraminotomy and the uncovertebral joints in the stability of the cervical spine. A biomechanical study. Spine (Phila Pa 1976). 1998; 23:1559–1565. PMID: 9682312.

17. Lebl DR, Hughes A, Cammisa FP Jr, O'Leary PF. Cervical spondylotic myelopathy : pathophysiology, clinical presentation, and treatment. HSS J. 2011; 7:170–178. PMID: 22754419.

18. Oh SH, Perin NI, Cooper PR. Quantitative three-dimensional anatomy of the subaxial cervical spine : implication for anterior spinal surgery. Neurosurgery. 1996; 38:1139–1144. PMID: 8727144.

19. White AA 3rd, Johnson RM, Panjabi MM, Southwick WO. Biomechanical analysis of clinical stability in the cervical spine. Clin Orthop Relat Res. 1975; (109):85–96. PMID: 1132209.

20. Wu XD, Yuan W, Chen HJ, Chen Y, Wang JX, Cao P, et al. Neck motion following multilevel anterior cervical fusion : comparison of short-term and midterm results. J Neurosurg Spine. 2013; 18:362–366. PMID: 23373566.

21. Yang HL, Chen GD, Zhang HT, Wang L, Luo ZP. Open-door laminoplasty with plate fixation at alternating levels for treatment of multilevel degenerative cervical disease. J Spinal Disord Tech. 2013; 26:E13–E18. PMID: 23075860.

22. Zhu B, Xu Y, Liu X, Liu Z, Dang G. Anterior approach versus posterior approach for the treatment of multilevel cervical spondylotic myelopathy : a systemic review and meta-analysis. Eur Spine J. 2013; 22:1583–1593. PMID: 23657624.

Fig. 1
Sketch diagram of measurement of vertebral body resection area (A), AP diameter (B), and transverse area (C) by TeraRecon Workstation.
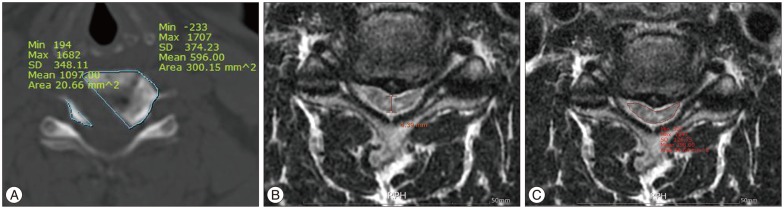
Fig. 2
A and B : Axial, T2-weighted, MRI scans demonstrated C4-5, C5-6 disc herniation with cervical cord compression. C : Sagittal, T2-weighted MRI. D : Sagittal, cervical CT showed ossification of posterior longitudinal ligaments. E and F : Axial, T2-weighted, MRI scans showed excellent decompression after surgery. G : Sagittal T2-weighted, MRI scans. H : Posto-perative cervical 3D CT showed microforaminotomy hole at C4-5, C5-6, Rt.

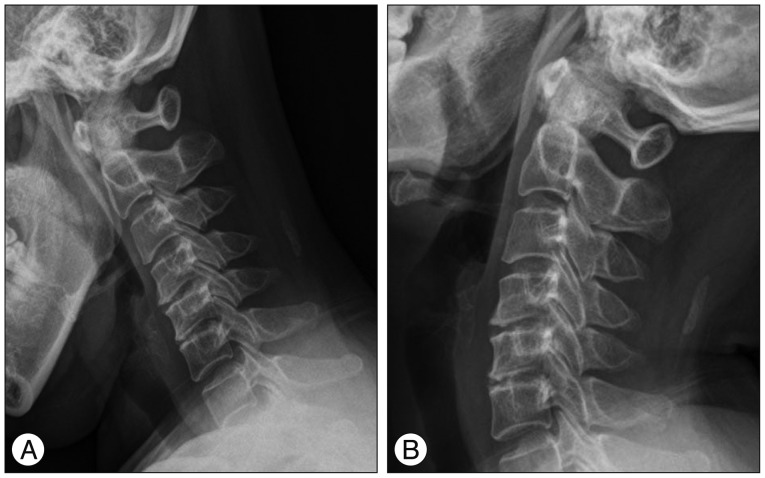
Fig. 3
Flexion (A) and extension (B) dynamic roentgenograms of the cervical spine, obtained 6 weeks after surgery, confirming normal motion at the C4-5, C5-6 level.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download