Abstract
Objective
Facial nerve palsy is a common complication of treatment for vestibular schwannoma (VS), so preserving facial nerve function is important. The preoperative visualization of the course of facial nerve in relation to VS could help prevent injury to the nerve during the surgery. In this study, we evaluate the accuracy of diffusion tensor tractography (DTT) for preoperative identification of facial nerve.
Methods
We prospectively collected data from 11 patients with VS, who underwent preoperative DTT for facial nerve. Imaging results were correlated with intraoperative findings. Postoperative DTT was performed at postoperative 3 month. Facial nerve function was clinically evaluated according to the House-Brackmann (HB) facial nerve grading system.
Results
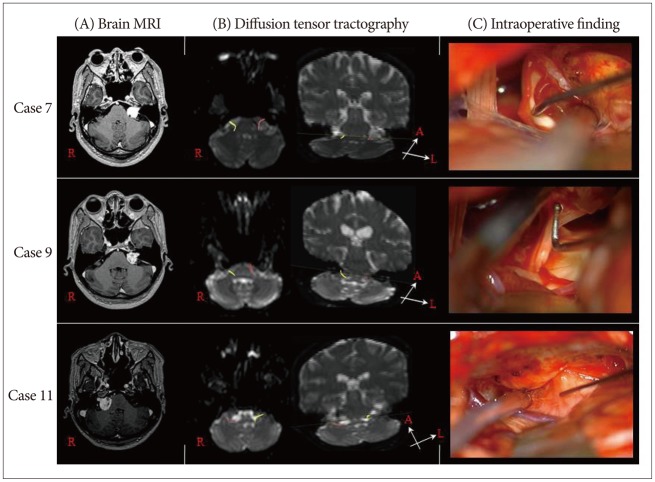
Facial nerve courses on preoperative tractography were entirely correlated with intraoperative findings in all patients. Facial nerve was located on the anterior of the tumor surface in 5 cases, on anteroinferior in 3 cases, on anterosuperior in 2 cases, and on posteroinferior in 1 case. In postoperative facial nerve tractography, preservation of facial nerve was confirmed in all patients. No patient had severe facial paralysis at postoperative one year.
Vestibular schwannomas (VS) is benign tumor which usually originates from the vestibular portion of the 8th cranial nerve. The removal of large VS by microsurgery is the gold standard of treatment. The goal of VS surgery is complete tumor removal as well as preservation of neurological functions of facial nerve20,21). Increasing expectations have paralleled improvements in techniques and technologies, and the introduction of intraoperative facial nerve monitoring1,6,11,13,16,23,27). However, a false-positive rate of 12-62% in intraoperative facial nerve monitoring has been reported18). Therefore, the predictive value for facial nerve function is limited.
Magnetic resonance (MR) imaging with constructive interference steady-state (CISS) sequences allows direct visualization of the cranial nerves in healthy patients2,10). Unfortunately, as these tumors enlarge and compress the facial nerve, preoperative identification using standard MR imaging becomes increasingly difficult. Recently, diffusion tensor tractography (DTT) derived from diffusion tensor imaging (DTI) has been demonstrated as feasible approach to show the position of cranial nerves in individual patients with VS3). Previous studies have reported that the preoperative prediction of the position of the facial nerve in relation to the VS could help prevent injury to the facial nerve during surgery using DTT3,4,19,26). This study aimed to investigate the accuracy of DTT for preoperative identification of the facial nerve through correlation with intraoperative findings.
We collected clinical data of 11 patients who underwent VS surgery between July 2011 and October 2012. The mean age of the patients was 51 years (range, 40-74), and the male-to-female ratio was 3 : 8. The initial symptom of most patients was asymmetric hearing impairment. Asymmetric sensorineural hearing loss was evident in seven patients (64%). Other initial symptoms were dizziness, tinnitus, and asymmetric facial paresthesia. The preoperative facial nerve function of all patients was normal. Tumor diameters ranged from 18 to 38 mm. Four patients were Koos grade II, two patients were grade III, and the other five patients were grade IV (Table 1)12).
All patients received preoperative DTT for the facial nerve and underwent operations using the retromastoid approach in the park bench position. All surgically treated patients underwent intraoperative facial nerve integrity monitoring, nerve stimulation, brainstem auditory evoked potential, electromyography and nerve conduction study. Postoperative DTT was performed at postoperative 3 month. Facial nerve function was clinically evaluated according to the House-Brackmann (HB) facial nerve grading system8).
DTI data were acquired using a 6-channel head coil on a 1.5 T Philips Gyroscan Intera (Philips, Ltd., Best, the Netherlands) with single-shot echo-planar imaging. For each of the 32 non-collinear diffusion sensitizing gradients, we acquired 70 contiguous slices parallel to the anterior commissure-posterior commissure line. Imaging parameters were as follows : acquisition matrix=96×96, reconstructed to matrix=192×192, field of view=240× 240 mm2, repetition time=10398 ms, echo time=72 ms, parallel imaging reduction factor (sensitivity encoding factor)=2, echo planar imaging factor=59 and b=1000 s/mm2, number of excitations=1, and a slice thickness of 2.5 mm (acquired isotropic voxel size 2.5×2.5×2.5 mm3). Affine multi-scale two-dimensional registration at the Oxford Centre for Functional Magnetic Resonance Imaging of Brain Software Library (FSL; www.fmrib.ox.ac.uk/fsl) was used for removal of eddy current-induced image distortions and motion artifacts24). DTI-Studio software (Center of Magnetic Resonance Microimaging, Johns Hopkins Medical Institute, Baltimore, MD, USA) was used for evaluation of the facial nerve tract9). Fiber tracking was based on the fiber assignment continuous tracking algorithm and a multiple regions of interest (ROIs) approach. For the reconstruction of facial nerve, two ROIs were drawn at the internal auditory meatus (IAM) and facial nerve entry area at the brainstem7,28). Fiber tracking was started at the center of a seed voxel with a fractional anisotropy >0.15 and ended at a voxel with a fractional anisotropy of <0.15 and a tract turning-angle of <70 degrees.
DTT was able to obtain a tract connecting the IAM and brainstem in all 11 cases. Facial nerve course on preoperative tractography were entirely correlated with intraoperative findings in all patients (100%). Facial nerve was located on the anterior of the tumor surface in 5 cases, on anteroinferior in 3 cases, on anterosuperior in 2 cases, and on posteroinferior in 1 case (Table 2).
The mean follow-up period was 20 months, with a range of 12-27 months. In postoperative facial nerve DTT, preservation of facial nerve was confirmed in all patients (Fig. 1). All patients were checked according to HB grade for facial nerve function at immediate postoperative 1 day. One patient was grade I, two patients were grade II, two patients were grade III, five patients were grade IV, and one patient was grade V. Patients with facial palsy were referred for intensive physiotherapy treatment. Facial nerve function at one year after surgery was clinically improved. Two patients recovered to HB grade III, seven patients recovered to HB grade II, and two other patients recovered HB grade I. Gross total resection (GTR) of tumor mass was achieved in 10 of the 11 cases (91%). Postoperative MRI revealed no residual mass in GTR group. In one patient (Case 10), the facial nerve was stretched thin and adhered to the tumor, thereby it was almost indistinguishable from the tumor mass. Therefore, we performed subtotal resection in order to preserve the facial nerve (Table 2).
Facial nerve preservation was evident in 10 of the 11 patients. In another patient (Case 2), the facial nerve was transected during tumor removal. Her facial nerve was very thin and fragile because of severe indentation caused by the tumor mass. We initially tried a primary end-to-end anastomosis, which failed due to the thinness and weakness the facial nerve. Thus, glue-coated collagen fleece (Tachocomb®) was used to connect the facial nerve.
Although evoked potential monitoring and careful visual inspections are performed to identify the compressed facial nerve during surgery, it is also desirable to obtain preoperative information about its course26). The reliable preoperative visualization of the nerve location in relation to the tumor may be valuable additional information for the surgeon. This information would allow the surgeon to plan the tumor removal accordingly and might increase the safety of the surgery4). However, despite the advances in surgical imaging, visualization of the three-dimensional anatomy of the lesion with its surrounding cranial nerves is, at times, difficult. Currently, the best methods of imaging of the CPA and its contents consist of two-dimensional MR imaging, including fast imaging employing steady-state acquisition and CISS methods17,25). Although the relationship of the lesion to its nearby cranial nerve can be visualized, detailed anatomy cannot be ascertained, particularly in cases of very large tumors5). Furthermore, thinned, splayed fibers, particularly of the facial/vestibular bundle, are often difficult to visualize because they course around the tumors. For larger lesions, it is difficult to visualize the facial nerve preoperatively using such conventional MR imaging22). This due in part to the nearly indistinguishable signal intensities of the facial nerve and VS, focal nerve thinning that occurs as a result of the tumor mass effect, and in large tumors, the lack of intervening cerebrospinal fluid between the two structures26).
Recently developed DTT allows the three-dimensional reconstruction of the cranial nerves in healthy individuals7). DTT is a novel modality of MR imaging analysis that measures the diffusion direction of water molecules by combining multiple diffusion-weighted image scans taken from multiple gradient directions. The diffusion of water molecules is thought to be anisotropic inside white matter tracts14,15) and therefore maximal along the direction of the fiber tracts14). A three-dimensional vector field (tensor) is assigned to each voxel. This information is then used to reconstruct and represent pictorially the white matter tracts within a specific ROI.
DTT reconstruction has been considered successful if a continuous tract of fibers is seen to extend from the IAM to the brainstem along the tumor capsule19). Taoka et al.26) applied these DTT techniques to patients with VS, and the authors reported that they were able to identify the location of the facial nerve in a subset of patients. We consider the technique of DTT to be a powerful tool for preoperatively predicting the course of the displaced facial nerve in VS. This information may increase the safety of surgery by enabling preservation of facial nerve function26). The reliable preoperative visualization of the nerve location in relation to the tumor may be valuable additional information for the surgeon. This information would allow the surgeon to plan the tumor removal accordingly and might increase the safety of the surgery4).
Hodaie et al.7) studied the course of cranial nerves using DT imaging-based tractography in 4 healthy patients. The study of the facial/vestibular complex with seeds in the internal auditory meatus primarily showed the cisternal segment of the nerves, and it was not possible to clearly differentiate the facial, vestibular, and cochlear components. Chen et al.3) successfully combined tractography and anatomical imaging to demonstrate the location of the trigeminal and facial nerves in 3 patients with VS and found that the facial nerve position was primarily anterior and inferior. In a study of 8 patients with VS, Taoka et al.26) were able to demonstrate a correspondence between the preoperative DT imaging reconstruction and the intraoperative location of the facial nerve in 5 patients (62.5%). Gerganov et al.4) proved that tractography provides highly reliable data : in 20 (90.9%) of 22 patients, a good correlation between the findings existed. And, recent study28) reported that preoperative identification of facial nerve was possible in 7 (87.5%) of 8 cases. The facial nerve location predicted by preoperative DTT agreed with surgical findings in all 7 cases. In this study, facial nerve courses on preoperative tractography were entirely correlated with intraoperative findings in all patients (100%).
Acknowledgements
This research was supported by a Grant of Yeungnam University Medical Center (2010).
References
1. Briggs RJ, Luxford WM, Atkins JS Jr, Hitselberger WE. Translabyrinthine removal of large acoustic neuromas. Neurosurgery. 1994; 34:785–790. discussion 790-791. PMID: 8052375.

2. Casselman JW, Kuhweide R, Deimling M, Ampe W, Dehaene I, Meeus L. Constructive interference in steady state-3DFT MR imaging of the inner ear and cerebellopontine angle. AJNR Am J Neuroradiol. 1993; 14:47–57. PMID: 8427111.
3. Chen DQ, Quan J, Guha A, Tymianski M, Mikulis D, Hodaie M. Three-dimensional in vivo modeling of vestibular schwannomas and surrounding cranial nerves with diffusion imaging tractography. Neurosurgery. 2011; 68:1077–1083. PMID: 21242825.

4. Gerganov VM, Giordano M, Samii M, Samii A. Diffusion tensor imaging-based fiber tracking for prediction of the position of the facial nerve in relation to large vestibular schwannomas. J Neurosurg. 2011; 115:1087–1093. PMID: 21962081.

5. Gjuric M, Rudic M. What is the best tumor size to achieve optimal functional results in vestibular schwannoma surgery? Skull Base. 2008; 18:317–325. PMID: 19240831.

6. Haberkamp TJ, Meyer GA, Fox M. Surgical exposure of the fundus of the internal auditory canal : anatomic limits of the middle fossa versus the retrosigmoid transcanal approach. Laryngoscope. 1998; 108(8 Pt 1):1190–1194. PMID: 9707242.

7. Hodaie M, Quan J, Chen DQ. In vivo visualization of cranial nerve pathways in humans using diffusion-based tractography. Neurosurgery. 2010; 66:788–795. discussion 795-796. PMID: 20305498.

8. House JW, Brackmann DE. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985; 93:146–147. PMID: 3921901.

9. Jiang H, van Zijl PC, Kim J, Pearlson GD, Mori S. DtiStudio : resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed. 2006; 81:106–116. PMID: 16413083.

10. Kakizawa Y, Seguchi T, Kodama K, Ogiwara T, Sasaki T, Goto T, et al. Anatomical study of the trigeminal and facial cranial nerves with the aid of 3.0-tesla magnetic resonance imaging. J Neurosurg. 2008; 108:483–490. PMID: 18312095.

11. Kartush JM. Intra-operative monitoring in acoustic neuroma surgery. Neurol Res. 1998; 20:593–596. PMID: 9785586.

12. Koos WT, Spetzler R. Color Atlas of Microneurosurgery: Microanatomy, Approaches, Techniques. ed 2. Stuttgart; New York: Thieme Medical Publishers;1993.
13. Lanman TH, Brackmann DE, Hitselberger WE, Subin B. Report of 190 consecutive cases of large acoustic tumors (vestibular schwannoma) removed via the translabyrinthine approach. J Neurosurg. 1999; 90:617–623. PMID: 10193604.

14. Mori S, van Zijl PC. Fiber tracking : principles and strategies - a technical review. NMR Biomed. 2002; 15:468–480. PMID: 12489096.
15. Mukherjee P, Chung SW, Berman JI, Hess CP, Henry RG. Diffusion tensor MR imaging and fiber tractography: technical considerations. AJNR Am J Neuroradiol. 2008; 29:843–852. PMID: 18339719.

16. Nadol JB Jr, Chiong CM, Ojemann RG, McKenna MJ, Martuza RL, Montgomery WW, et al. Preservation of hearing and facial nerve function in resection of acoustic neuroma. Laryngoscope. 1992; 102:1153–1158. PMID: 1405966.

17. Naganawa S, Koshikawa T, Fukatsu H, Ishigaki T, Fukuta T. MR cisternography of the cerebellopontine angle : comparison of three-dimensional fast asymmetrical spin-echo and three-dimensional constructive interference in the steady-state sequences. AJNR Am J Neuroradiol. 2001; 22:1179–1185. PMID: 11415916.
18. Neff BA, Ting J, Dickinson SL, Welling DB. Facial nerve monitoring parameters as a predictor of postoperative facial nerve outcomes after vestibular schwannoma resection. Otol Neurotol. 2005; 26:728–732. PMID: 16015176.

19. Roundy N, Delashaw JB, Cetas JS. Preoperative identification of the facial nerve in patients with large cerebellopontine angle tumors using high-density diffusion tensor imaging. J Neurosurg. 2012; 116:697–702. PMID: 22283188.

20. Samii M, Gerganov V, Samii A. Improved preservation of hearing and facial nerve function in vestibular schwannoma surgery via the retrosigmoid approach in a series of 200 patients. J Neurosurg. 2006; 105:527–535. PMID: 17044553.

21. Samii M, Gerganov VM, Samii A. Functional outcome after complete surgical removal of giant vestibular schwannomas. J Neurosurg. 2010; 112:860–867. PMID: 19663543.

22. Sartoretti-Schefer S, Kollias S, Valavanis A. Spatial relationship between vestibular schwannoma and facial nerve on three-dimensional T2-weighted fast spin-echo MR images. AJNR Am J Neuroradiol. 2000; 21:810–816. PMID: 10815653.
23. Sluyter S, Graamans K, Tulleken CA, Van Veelen CW. Analysis of the results obtained in 120 patients with large acoustic neuromas surgically treated via the translabyrinthine-transtentorial approach. J Neurosurg. 2001; 94:61–66. PMID: 11147899.

24. Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004; 23(Suppl 1):S208–S219. PMID: 15501092.

25. Stuckey SL, Harris AJ, Mannolini SM. Detection of acoustic schwannoma : use of constructive interference in the steady state three-dimensional MR. AJNR Am J Neuroradiol. 1996; 17:1219–1225. PMID: 8871702.
26. Taoka T, Hirabayashi H, Nakagawa H, Sakamoto M, Myochin K, Hirohashi S, et al. Displacement of the facial nerve course by vestibular schwannoma : preoperative visualization using diffusion tensor tractography. J Magn Reson Imaging. 2006; 24:1005–1010. PMID: 17031835.

27. Wu H, Sterkers J. Translabyrinthine removal of large acoustic neuromas in young adults. Auris Nasus Larynx. 2000; 27:201–205. PMID: 10808105.

28. Zhang Y, Chen Y, Zou Y, Zhang W, Zhang R, Liu X, et al. Facial nerve preservation with preoperative identification and intraoperative monitoring in large vestibular schwannoma surgery. Acta Neurochir (Wien). 2013; 155:1857–1862. PMID: 23877233.

Fig. 1
Case 7 : facial nerve is located on the anteroinferior of the tumor surface. Case 9 : facial nerve is located on the anterior of the tumor surface. Case 11 : facial nerve is located on the posteroinferior of the tumor surface. A : The preoperative axial enhanced T1-weighted magnetic resonance image shows a vestibular schwannoma. B : The preoperative diffusion tensor imaging of facial nerve tractography. C : Intraoperative findings.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download