1. Abe T, Morishige M, Ooba H, Kamida T, Fujiki M, Kobayashi H, et al. The association between high VEGF levels and multiple probable punctuate cavernous malformations. Acta Neurochir (Wien). 2009; 151:855–859. PMID:
19479188.

2. Cakirer S. De novo formation of a cavernous malformation of the brain in the presence of a developmental venous anomaly. Clin Radiol. 2003; 58:251–256. PMID:
12639533.

3. Campeau NG, Lane JI. De novo development of a lesion with the appearance of a cavernous malformation adjacent to an existing developmental venous anomaly. AJNR Am J Neuroradiol. 2005; 26:156–159. PMID:
15661718.
4. Garner TB, Del Curling O Jr, Kelly DL Jr, Laster DW. The natural history of intracranial venous angiomas. J Neurosurg. 1991; 75:715–722. PMID:
1919693.

5. Jung KH, Chu K, Jeong SW, Park HK, Bae HJ, Yoon BW. Cerebral cavernous malformations with dynamic and progressive course : correlation study with vascular endothelial growth factor. Arch Neurol. 2003; 60:1613–1618. PMID:
14623736.

6. Kim YS, Lee JI, Choi CH, Ko JK. Massive intracerebral hemorrhage caused by a cavernous malformation. J Korean Neurosurg Soc. 2012; 51:37–39. PMID:
22396841.

7. Kondziolka D, Lunsford LD, Kestle JR. The natural history of cerebral cavernous malformations. J Neurosurg. 1995; 83:820–824. PMID:
7472549.

8. Massa-Micon B, Luparello V, Bergui M, Pagni CA. De novo cavernoma case report and review of literature. Surg Neurol. 2000; 53:484–487. PMID:
10874148.

9. Masuzawa T, Saito K, Shimabukuro H, Iwasa H, Sato F. Chronic encapsulated hematomas in the brain. Acta Neuropathol. 1985; 66:24–28. PMID:
3993333.

10. Miyahara K, Fujitsu K, Yagishita S, Ichikawa T, Takemoto Y, Okada T, et al. Chronic encapsulated intracerebral hematoma associated with cavernous angioma--case report. Neurol Med Chir (Tokyo). 2011; 51:52–55. PMID:
21273746.

11. Motegi H, Kuroda S, Ishii N, Aoyama H, Terae S, Shirato H, et al. De novo formation of cavernoma after radiosurgery for adult cerebral arteriovenous malformation--case report. Neurol Med Chir (Tokyo). 2008; 48:397–400. PMID:
18812682.

12. Murakami S, Sotsu M, Morooka S, Suzuki T. Chronic encapsulated intracerebral hematoma associated with cavernous angioma : a case report. Neurosurgery. 1990; 26:700–702. PMID:
2330096.

13. Nakamizo A, Suzuki SO, Saito N, Shono T, Matsumoto K, Onaka S, et al. Clinicopathological study on chronic encapsulated expanding hematoma associated with incompletely obliterated AVM after stereotactic radiosurgery. Acta Neurochir (Wien). 2011; 153:883–893. PMID:
20931239.

14. Roda JM, Carceller F, Pérez-Higueras A, Morales C. Encapsulated intracerebral hematomas : a defined entity. Case report. J Neurosurg. 1993; 78:829–833. PMID:
8468616.
15. Takeuchi S, Nawashiro H, Wada K, Minamimura K. Chronic encapsulated intracerebral hematoma associated with cavernous angioma : expression of vascular endothelial growth factor and its receptor. Neurol India. 2011; 59:903–904. PMID:
22234209.

16. Takeuchi S, Takasato Y. Chronic encapsulated intracerebral hematoma : a rare complication after stereotactic radiosurgery for cerebral arteriovenous malformation. Acta Neurochir (Wien). 2011; 153:895. PMID:
21052741.

17. Takeuchi S, Takasato Y, Masaoka H. Chronic encapsulated intracerebral hematoma formation after radiosurgery for cerebral arteriovenous malformation. Neurol India. 2011; 59:624–626. PMID:
21891948.

18. Takeuchi S, Takasato Y, Masaoka H, Hayakawa T, Otani N, Yoshino Y, et al. Development of chronic encapsulated intracerebral hematoma after radiosurgery for a cerebral arteriovenous malformation. Acta Neurochir (Wien). 2009; 151:1513–1515. PMID:
19597762.

19. Washington CW, McCoy KE, Zipfel GJ. Update on the natural history of cavernous malformations and factors predicting aggressive clinical presentation. Neurosurg Focus. 2010; 29:e7. PMID:
20809765.

20. Yeon JY, Suh YL, Kim JH, Lee JI. Development of de novo cavernous hemangioma after radiosurgery for cavernous hemangioma. J Korean Neurosurg Soc. 2010; 48:532–533. PMID:
21430981.

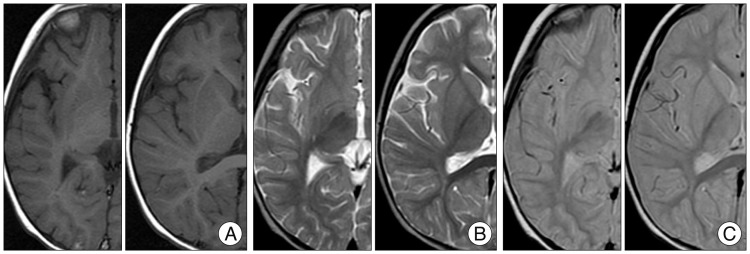
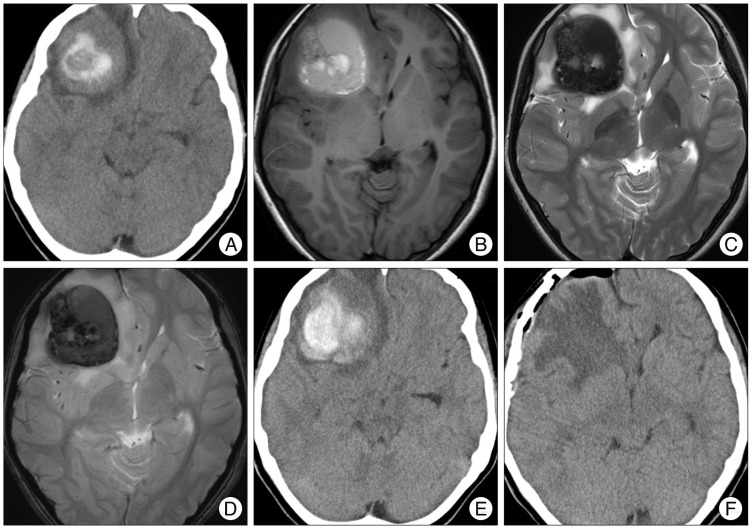
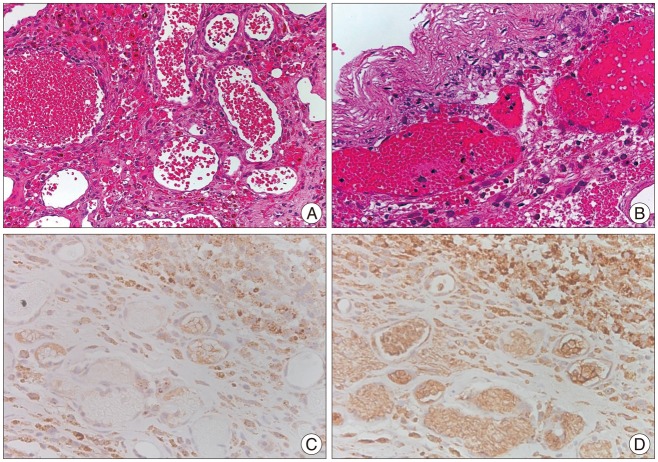




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download