Abstract
Ehlers-Danlos syndrome (EDS) is a rare inherited connective disease. Among several subgroups, type IV EDS is frequently associated with spontaneous catastrophic bleeding from a vascular fragility. We report on a case of carotid-cavernous fistula (CCF) in a patient with type IV EDS. A 46-year-old female presented with an ophthalmoplegia and chemosis in the right eye. Subsequently, seizure and cerebral infarction with micro-bleeds occurred. CCF was completely occluded with transvenous coil embolization without complications. Thereafter, the patient was completely recovered. Transvenous coil embolization can be a good treatment of choice for spontaneous CCF with type IV EDS. However, every caution should be kept during invasive procedure.
Ehlers-Danlos syndrome (EDS) is a dominantly inherited connective tissue disorder characterized by abnormal collagen synthesis2,22). The latest classification consisted of 6 major subtypes depending on clinical and genetic features5,8). Thin and translucent skin, easy bruisability, and intestinal/uterine/vascular fragility are characteristic features of type IV EDS (vascular type or arterial ecchymotic type). Type IV EDS is frequently associated with spontaneous catastrophic bleeding due to generalized vascular fragility6,8,9,13). About 50% of the affected individuals have the COL3A1 gene mutation which is associated with abnormal efficiency of type III procollagen secretion16,17,18). Spontaneous carotid-cavernous fistula is one of well-known complications in patients with type IV EDS14,15,16,22). Here, we report a case of carotid-cavernous fistula (CCF) with type IV EDS, which was treated by transvenous coiling embolization of the fistula.
A 46-year-old female presented with diplopia and conjunctival ecchymosis for 5 days. She had suffered from headache and pulsatile tinnitus on the right side for 2 months. She had repeated medical histories of easy bruisability, rupture of the tendon and ligament as well as the subluxation of multiple joints. She had a thin, translucent, and fragile skin with prominent veins, especially on the hands. Ophthalmologic evaluation showed chemosis with mild exophthalmia and lateral gaze limitation on the right side, without any problem of visual acuity or field. She had a senile-looking face, hair loss, metacarpophalangeal joint dislocation, radial head dislocation at extension on the right side, distal radioulnar joint instability, fourth brachymetatarsia, and Lisfranc (tarsometatarsal) joint dissociations bilaterally (Fig. 1). Two daughters of 22 and 17 years old showed similar appearances (Fig. 2). Brain magnetic resonance (MR) angiography demonstrated the CCF on the right side associated with the right superior ophthalmic vein (SOV) dilatation. Thoracoabdominal computed tomographic angiography demonstrated multifocal splenic artery aneurysms. On genetic investigation, her COL3A1 gene mutation was identified by polymerase chain reaction assays. A splice site mutation in intron 23 of one allele of the COL3A1 gene [c. 1662+1 (intervening sequence 24) G→A] was found to be the causative mutation. Her daughters had the same genotype. Because of the high risk of vascular damage from conventional angiography and intervention, further investigation and treatment was deferred.
Two months later, she was readmitted due to the generalized tonic-clonic seizure. Brain MR imaging revealed venous infarction at the right anterior insular cortex and right frontal lobe with micro-bleeds on gradient-echo images (Fig. 3). As we considered these features as ominous signs of impending intracranial hemorrhage and poor prognosis, the decision was made to precede embolization of CCF. Under the ultrasonographic guidance, her femoral artery was carefully punctured and 4 Fr sheath was introduced. Then, cerebral angiography was carefully performed with a 4 Fr angiocatheter. On angiography, direct CCF accompanied multiple venous drainage into the right SOV, right inferior petrosal sinus (IPS), and right cavernous sinus (CS) with reflux to the superficial middle cerebral vein and medullary veins (Fig. 4A). Notably, the right IPS was stenosed. On right common carotid artery angiography with the left neck compression, fistular point was identified at the medial compartment of CS.
After the angiographic evaluation, a 5 Fr guiding catheter was positioned at the right internal jugular vein via femoral vein. A microcatheter was placed at the right CS near the proximal portion of right SOV via the right IPS. The CS near the fistular point was completely embolized with fibered coils. There were no fistulous flow and venous reflux, and normal antegrade flow through the right internal carotid artery (ICA) was restored with subtle venous hyperemia on the perisylvian area (Fig. 4B). The clinical course was uneventful after the embolization. Bruit disappeared and right eye movement improved at discharge. Chemosis, proptosis and limitation of the ocular motion were completely resolved on 6-month follow-up after the transvenous embolization.
Direct CCFs are abnormal communications between the intracavernous ICA and CS. Head trauma is the most common cause1,7). Spontaneous non-traumatic direct CCFs usually develop in patients with intracavernous ICA aneurysms or connective tissue diseases such as EDS1,7,14,15,16). EDS is a heterogenous group of connective tissue disorders affecting 1/500000 to 1/5000 individuals5). The classification consists of 6 major subtypes depending on clinical and genetic features (Table 1)2,3,4). The diagnosis can be made clinically using the criteria formulated by a medical advisory group (Table 2). The presence of 2 or more of the major criteria (thin translucent skin; arterial, intestinal or uterine fragility or rupture; extensive bruising; characteristic facial appearance) is highly indicative for EDS and the laboratory testing is strongly recommended4).
Type IV (vascular type) is the rarest (4%) among the subgroups, however, it is the severest form of EDS15,20). Although there are significantly inter- and intrafamilial variabilities in the clinical presentation, the age of manifestation ranges from 25 to 28 years old and most of patients lead to death at the age of 45 to 50 years old, often due to arterial rupture. According to a recent review of 419 patients of type IV EDS16), the first presenting age of vascular or visceral complication is about 24 years with a mortality rate of 12%, and cumulative risk of major complications is 80% by age 40. In addition, 70% of deaths resulted from the rupture of arteries, mostly thoracic and abdominal vessels as well as cerebral vessels. The most common nonlethal vascular complication in that series is the direct type CCF. The cause of CCF associated with type IV EDS is considered to be rupture of a preexisting intracavernous ICA aneurysm. Weakness of ICA wall is associated with the aneurysmal formation and subsequent rupture leads to the formation of CCF11). Other complications include the rupture of colon and uterus during pregnancy. Pregnancy carries a mortality rate of 11% from arterial or uterine rupture16).
EDS type IV is genetically distinct from other types of EDS. The gene encoding procollagen III is located on chromosome 2 (2q31-q32.3)19,23). While skin fibroblasts usually synthesize both types I and III collagen, those in patients with type IV EDS synthesize only type I. Type III procollagen normally accounts for 6% to 20% of the total collagenous proteins secreted into the medium by the normal fibroblast. The absence of type III procollagen in type IV EDS results in defective skin, bowel, stomach, and blood vessels20). The diagnosis can be confirmed by analysis of type III procollagen produced by cultured fibroblasts or detection of the mutation in the COL3A1 gene17). A total number of reported mutation of COL3A1 gene is 23925). In our case, splicing mutation was identified. This mutation has been reported already by Schwarze in 199723). Once a mutation is identified, other family members are to be screened16).
Transarterial angiography has been known to be associated with high risk of arterial complications, such as rupture and dissection of aortic, renal, hepatic, subclavian, splenic, and iliac arteries, even though the diagnosis had been established prior to angiography and appropriate precautions are taken14). The morbidity and mortality were respectively 36% and 12% in 25 angiographic procedures in patients with type IV EDS21). Thus, diagnostic procedures requiring arterial punctures should be avoided, except in indispensible situations. Patients with probable type IV EDS warrant a low-risk investigative approach. Transvenous angiography or MR imaging are advocated as the initial investigative method. In the present case, initial definitive investigation and treatment was deferred for these reasons. Angiography and embolization of the CCF was performed when the patient worsened with the imaging evidence of a high risk of intracranial hemorrhage.
Since Serbinenko24) described treatment of CCF with detachable balloons in 1971, endovascular treatment has been the treatment of choice for CCF. However, even endovascular treatment frequently resulted in poor outcomes. Farley et al.7) reported a fatal outcome after balloon embolization in a patient with CCF and type IV EDS. The patient died of pontine hemorrhage 4 days after embolization. Lach et al.14) reported another mortality case of right common iliac artery laceration 15 days after balloon embolization. Halbach et al.9) reported 33% mortality with balloon embolization in type IV EDS patients with CCF. Placement of a grafted stent in the cavernous ICA covering the fistular point might be a treatment option. However, this approach has never been used for treatment of CCF in type IV EDS patients. Because the vascular wall is fragile and the stent delivery system is very rigid, this approach would carry a high risk of rupture of the blood vessel. Third option is trapping of the ICA including fistula if the ipsilateral hemisphere has abundant collateral circulation. We believe that it could be the last resort in an inevitable situation. Finally, coil embolization can be a good alternative. Transvenous coil embolization, the method chosen for the present case, has been rarely reported12). We believe that coils are adequate material in this condition because they are soft and easy to control. Moreover, they allow the use of smaller delivery devices for insertion and exert less pressure on the CS and ICA than balloons do. In addition, the advantage of transvenous approach is that iatrogenic injury to a low-pressure vein might be less deleterious than injury to a high-pressure artery. Nonetheless, reported periprocedural complications with transvenous coil embolization have been substantial, including retroperitoneal hematoma, colonic rupture, and fatal iliac artery perforation2,10). Fortunately, our patient recovered well with no complications. However, even when no procedural complications are noted, these patients need specific and extensive monitoring in a specialized intensive care unit after interventional treatment.
We described a case of CCF with type IV EDS, safely treated with transvenous coil embolization. This exemplary case shows that successful endovascular treatment of symptomatic CCF in patients with EDS is possible. However, we need to keep in mind that it is a challenging condition given the potential risk of lethal procedure-related complications, mostly hemorrhagic.
References
1. Barrow DL, Spector RH, Braun IF, Landman JA, Tindall SC, Tindall GT. Classification and treatment of spontaneous carotid-cavernous sinus fistulas. J Neurosurg. 1985; 62:248–256. PMID: 3968564.

2. Bashir Q, Thornton J, Alp S, Debrun GM, Aletich VA, Charbel F, et al. Carotid-cavernous fistula associated with Ehlers-Danlos syndrome type IV. A case report and review of literature. Interv Neuroradiol. 1999; 5:313–320. PMID: 20670529.

3. Beighton P, de Paepe A, Danks D, Finidori G, Gedde-Dahl T, Goodman R, et al. International Nosology of Heritable Disorders of Connective Tissue, Berlin, 1986. Am J Med Genet. 1988; 29:581–594. PMID: 3287925.

4. Beighton P, De Paepe A, Steinmann B, Tsipouras P, Wenstrup RJ. Ehlers-Danlos National Foundation (USA) and Ehlers-Danlos Support Group (UK). Ehlers-Danlos syndromes : revised nosology, Villefranche, 1997. Am J Med Genet. 1998; 77:31–37. PMID: 9557891.
5. Burrows NP. The molecular genetics of the Ehlers-Danlos syndrome. Clin Exp Dermatol. 1999; 24:99–106. PMID: 10233664.

6. Debrun GM, Aletich VA, Miller NR, DeKeiser RJ. Three cases of spontaneous direct carotid cavernous fistulas associated with Ehlers-Danlos syndrome type IV. Surg Neurol. 1996; 46:247–252. PMID: 8781594.

7. Farley MK, Clark RD, Fallor MK, Geggel HS, Heckenlively JR. Spontaneous carotid-cavernous fistula and the Ehlers-Danlos syndromes. Ophthalmology. 1983; 90:1337–1342. PMID: 6664673.

8. Forlodou P, de Kersaint-Gilly A, Pizzanelli J, Viarouge MP, Auffray-Calvier E. Ehlers-Danlos syndrome with a spontaneous caroticocavernous fistula occluded by detachable balloon : case report and review of literature. Neuroradiology. 1996; 38:595–597. PMID: 8880727.

9. Halbach VV, Higashida RT, Dowd CF, Barnwell SL, Hieshima GB. Treatment of carotid-cavernous fistulas associated with Ehlers-Danlos syndrome. Neurosurgery. 1990; 26:1021–1027. PMID: 2362658.

10. Horowitz MB, Purdy PD, Valentine RJ, Morrill K. Remote vascular catastrophes after neurovascular interventional therapy for type 4 Ehlers-Danlos Syndrome. AJNR Am J Neuroradiol. 2000; 21:974–976. PMID: 10815681.
11. Julien I, De Boucaud D. [Spontaneous carotid-cavernous fistula and Ehlers-Danlos diseases]. Presse Med. 1971; 79:1241–1242. PMID: 5556792.
12. Kanner AA, Maimon S, Rappaport ZH. Treatment of spontaneous carotid-cavernous fistula in Ehlers-Danlos syndrome by transvenous occlusion with Guglielmi detachable coils. Case report and review of the literature. J Neurosurg. 2000; 93:689–692. PMID: 11014550.

13. Kashiwagi S, Tsuchida E, Goto K, Shiroyama Y, Yamashita T, Takahasi M, et al. Balloon occlusion of a spontaneous carotid-cavernous fistula in Ehlers-Danlos syndrome type IV. Surg Neurol. 1993; 39:187–190. PMID: 8456380.

14. Lach B, Nair SG, Russell NA, Benoit BG. Spontaneous carotid-cavernous fistula and multiple arterial dissections in type IV Ehlers-Danlos syndrome. Case report. J Neurosurg. 1987; 66:462–467. PMID: 3819843.

15. North KN, Whiteman DA, Pepin MG, Byers PH. Cerebrovascular complications in Ehlers-Danlos syndrome type IV. Ann Neurol. 1995; 38:960–964. PMID: 8526472.

16. Pepin M, Schwarze U, Superti-Furga A, Byers PH. Clinical and genetic features of Ehlers-Danlos syndrome type IV, the vascular type. N Engl J Med. 2000; 342:673–680. PMID: 10706896.

17. Pope FM, Martin GR, Lichtenstein JR, Penttinen R, Gerson B, Rowe DW, et al. Patients with Ehlers-Danlos syndrome type IV lack type III collagen. Proc Natl Acad Sci U S A. 1975; 72:1314–1316. PMID: 1055406.

18. Pope FM, Nicholls AC, Narcisi P, Temple A, Chia Y, Fryer P, et al. Type III collagen mutations in Ehlers Danlos syndrome type IV and other related disorders. Clin Exp Dermatol. 1988; 13:285–302. PMID: 3076851.

19. Povey S, Falk CT. Report of the committee on the genetic constitution of chromosome 2. Cytogenet Cell Genet. 1989; 51:91–105. PMID: 2676388.

20. Schievink WI, Michels VV, Piepgras DG. Neurovascular manifestations of heritable connective tissue disorders. A review. Stroke. 1994; 25:889–903. PMID: 8160237.

21. Schievink WI, Piepgras DG, Earnest F 4th, Gordon H. Spontaneous carotid-cavernous fistulae in Ehlers-Danlos syndrome Type IV. Case report. J Neurosurg. 1991; 74:991–998. PMID: 2033461.
22. Schoolman A, Kepes JJ. Bilateral spontaneous carotid-cavernous fistulae in Ehlers-Danlos syndrome. Case report. J Neurosurg. 1967; 26:82–86. PMID: 6018786.

23. Schwarze U, Goldstein JA, Byers PH. Splicing defects in the COL3A1 gene : marked preference for 5' (donor) spice-site mutations in patients with exon-skipping mutations and Ehlers-Danlos syndrome type IV. Am J Hum Genet. 1997; 61:1276–1286. PMID: 9399899.

24. Serbinenko FA. Occlusion of the cavernous portion of the carotid artery with a balloon as a method of treating carotid-cavernous anastomosis. Vopr Neirokhir. 1971; 35:3–9. PMID: 5147941.
25. The Human Gene Mutation Database. accessed on January 14, 2013. Available at : http://www.hgmd.cf.ac.uk/ac/gene.php?gene=COL3A1.
Fig. 1
Gross photograph. Skin appearance is typical, with thin, translucent, and fragile skin with a prominent venous pattern, especially on the hands.
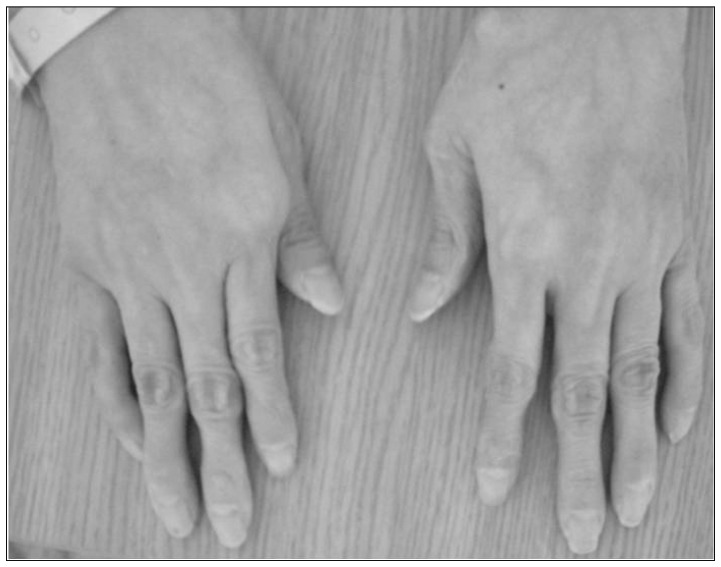
Fig. 2
Pedigree. The sister of the proband (black arrow)2) was known to have a COL3A1 mutation of Ehlers-Danlos type IV. She had had easy bruisability and a spontaneous carotid cavernous fistula. Her two daughters were confirmed by DNA sequencing. EDS : Ehlers-Danlos syndrome.
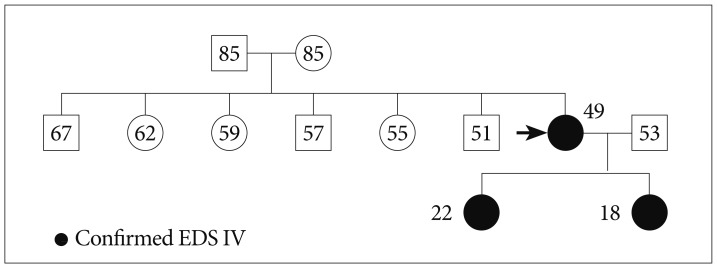
Fig. 3
Brain magnetic resonance imaging shows venous infarction at the right anterior insular cortex and right frontal lobe (A) with micro-bleedings on gradient-echo images (B).
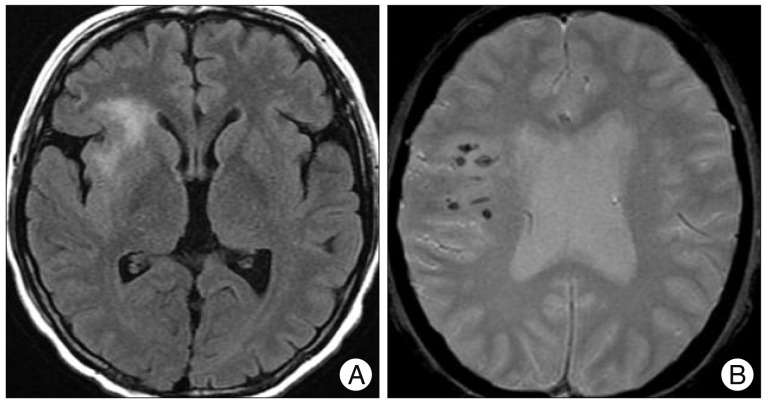
Fig. 4
Cerebral angiography. A : Pre-embolization right internal carotid artery (ICA) angiography shows a direct carotid-cavernous fistula with reduced intracranial flow to the distal ICA. B : On post-embolization angiography, fistula is completely occluded with no reflux into the superficial middle cerebral vein. Normal antegrade flow through the right ICA is restored with subtle venous hyperemia remaining in the perisylvian area.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download