Abstract
A differential diagnosis between neurosarcoidosis and neurosyphilis is particularly problematic in patients with a positive serologic result for syphilis. We report here a patient with a solitary cavernous sinus sarcoidosis who had a history of syphilis and showed rapidly progressing cavernous sinus syndrome. A transsphenoidal biopsy was performed and a histopathologic examination revealed a non-caseating granuloma with an asteroid body. His facial pain disappeared after steroid therapy. He received oral prednisolone for one year. A follow-up magnetic resonance imaging of the brain revealed resolution of the mass over the cavernous sinus. Particularly in patients with a history of syphilis, neurosyphilis should be included in a differential diagnosis of neurosarcoidosis.
Sarcoidosis is a granulomatous multisystem disorder with an unknown etiology. Sarcoidosis mainly involves the lungs and thoracic lymph nodes but can affect almost any organs including the central nervous system (CNS) and leptomeninges20). Nevertheless, a solitary neurosarcoidosis is rare and is estimated to occur in approximately 5-15% of all patients affected by sarcoidosis5,8,23).
A definitive diagnosis of neurosarcoidosis requires the exclusion of alternative diseases22,23). Neurological diseases (multiple sclerosis, acute demyelinating encephalomyelitis), infectious diseases (tuberculosis, cryptococcosis, neurosyphilis), neoplasms (lymphoma, en plaque meningioma, metastatic carcinoma, primary CNS tumor), and vasculitis (Wegener's granulomatosis, Churg-strauss syndrome) can be included in differential diagnosis20). A differential diagnosis between neurosarcoidosis and neurosyphilis is particularly problematic in patients with a positive serologic result for syphilis.
We report the case of a patient with a solitary cavernous sinus sarcoidosis who had a history of syphilis and showed rapidly progressing cavernous sinus syndrome.
A 40-year-old man experienced the sudden onset of diplopia one month before admission. At that time, he was evaluated for his ophthalmoplegia in an outside hospital with brain magnetic resonance imaging (MRI), which demonstrated right sphenoid and left posterior ethmoid sinusitis. One month after the onset of diplopia, he still suffered from diplopia. He also developed a new onset of right facial pain and headache. The follow-up MRI revealed an enhancing mass lesion in the right cavernous sinus region. He was referred to our neurosurgical unit with the presumptive diagnosis of a cavernous sinus tumor.
He had a history of syphilis of unknown stage, which had been treated 20 years earlier. At admission, he was alert and fully oriented. He presented with diplopia, headache, right facial pain, and mild dysarthria. The neurological examination was notable for the complete palsy of abduction in his right eye, paresthesia in all divisions of the right trigeminal nerve and right facial palsy with mild dysarthria. There was no tinnitus, uvula deviation, or tongue deviation. In serum laboratory examination, his venereal disease research laboratory (VDRL) titer was 1 : 16. Treponema pallidum hemagglutination and fluorescent treponemal antibody absorption test were reactive. The serum angiotension-converting enzyme (ACE) level was 12.5 IU/L (normal range : 8.3-21.4 IU/L) and the HIV test was negative. The cerebrospinal fluid (CSF) examination revealed a normal opening pressure at lumbar puncture, 20 white blood cells (80% lymphocytes), no red blood cells, protein level of 41 mg%, and glucose level of 79 mg%. The CSF VDRL test was negative.
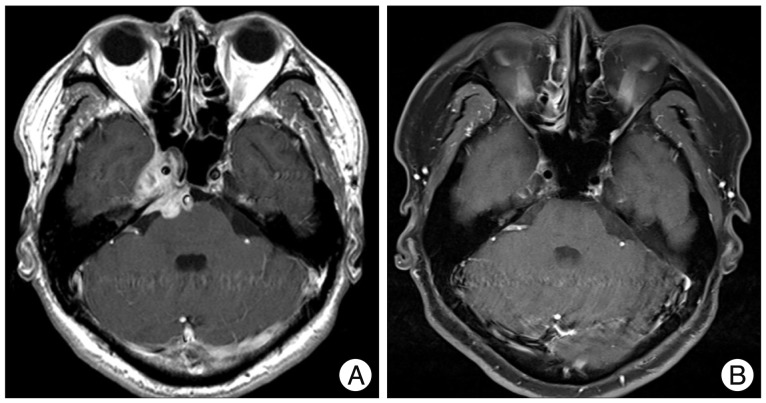
Brain MRI demonstrated a lobulated mass predominantly involving the right cavernous sinus (Fig. 1A). The mass extended toward the middle fossa laterally and to the sphenoid sinus anteriorly. This tumor also extended to the cerebellopontine angle posteriorly with a compression of the brain stem. The lesion appeared isointense on T1-weighted images and slightly hyperintense on the T2-weighted images. Moreover, the T2-weighted images revealed hyperintense signal changes in the pontine area. The mass showed strong enhancement after gadolinium administration. On the other hand, malignancy and inflammatory disorders were included in the differential diagnosis considering the rapid enlargement of mass and serological markers.
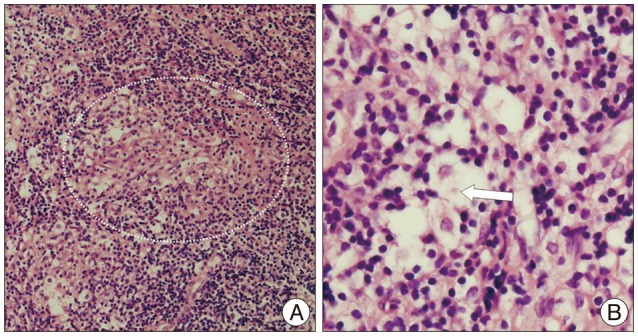
A biopsy was performed via the transsphenoidal approach to make a precise diagnosis. The surgical approach was chosen based on the protrusion of pathology in the sphenoidal sinus and minimal invasive technique. A histopathological examination revealed a non-caseating granuloma with an asteroid body (Fig. 2). Special stains including periodic acid-Schiff, Ziehl-Neelsen stain for acid-fast bacilli and Gomori's methenamine silver stain for fungal organisms were negative. Chest and abdomen computed tomography were performed to exclude systemic sarcoidosis, but unremarkable.
After administration of corticosteroid, the headache and facial paresthesia was improved but the diplopia has remained. The pathologic findings were consistent with a diagnosis of sarcoidosis, but the neurosyphilis could not be ruled out in the differential diagnosis due to the positive serologic result for syphilis. The patient received the treatment with intravenous penicillin G for 2 weeks and the steroid was tapered and discontinued. After 4 days of penicillin administration, the right side facial pain and paresthesia became aggravated. Therefore, we were confident in a diagnosis of neurosarcoidosis and resumed the steroid treatment. The facial pain disappeared again after steroid therapy. The steroid dose was tapered down and shifted to oral prednisolone in the third week. He was discharged with isolated right abducens nerve palsy. He received oral prednisolone at the outpatient department for one year and the abducens nerve palsy was improved. A follow-up MRI of the brain revealed resolution of the mass over the cavernous sinus region (Fig. 1B).
Sarcoidosis is an idiopathic system disorder with an unknown origin that can affect almost every organ in the body, including the CNS. The lung and reticuloendoendothelial system are typically involved, but any organs can be affected17). Sarcoidosis involving the CNS is relatively uncommon, occurring in about 5-15% of patients with sarcoidosis5,8,11).
The laboratory findings of neurosarcoidosis are generally non-specific and are not present in all patients. CSF examination in neurosarcoidosis reveals a lymphocytic pleocytosis, low glucose, and high protein concentrations, high opening pressure or evidence of intrathecal immunoglobulin synthesis in some patients9,23). The serum ACE levels can be elevated in patients with sarcoidosis but the levels are usually normal in isolated neurosarcoidosis18). In addition, the CSF ACE levels are relatively specific (94-95%), but insensitive (24-55%)10).
The imaging procedure of choice for neurosarcoidosis is MRI without and with gadolinium8,20). The range of abnormalities included white matter lesions, hydrocephalus, mass lesions in the brain parenchyma, meningeal enhancement, enhancement of parenchymal lesions and lesions of the optic nerves and spinal cord, with or without enlargement of these structures23). Because of the leptomeningeal involving nature of sarcoidosis, leptomeningeal enhancement with parenchymal periarterial enhancement is common and distinguishing abnormality in neurosarcoidosis11).
The histopathology of sarcoidosis is characteristic. A pathologic specimen normally reveals the presence of non-caseating granulomas observed in the absence of a foreign body reaction or tuberculous infection, giant multinucleated (epitheloid) cells and macrophages surrounded by an inflammatory reaction4,21). In addition, an asteroid body can be observed, particularly in sarcoidosis1).
Minimally aggressive biopsy is desirable, because neurosurgical resection of intracranial granulomas is only indicated in life-threatening situation or when medical treatment is insufficient. The optimal treatment for all sarcoidosis is the chronic administration of corticosteroid. In spite of significant side-effect, corticosteroids are mostly effective in neurosarcoidosis4,23).
The diagnosis of neurosyphilis is also difficult because of the lack of a perfect gold standard. The non-treponemal CSF VDRL test is a mainstay for diagnosis of neurosyphilis12). Although the estimated specificity of the test is high, the sensitivity is lower, which is a major limitation of this test12). Furthermore, the CSF VDRL test is negative in 50% of neurosyphilis cases. Moreover, a negative CSF treponemal-specific antibody test may not exclude a diagnosis of neurosyphilis in cases where the clinical suspicion is high6).
Neurosyphilis has similar histopathologic and etiologic features to neurosarcoidosis. Neurosyphilis include gummas, meningovascular inflammation, inflammation of the cerebral vessels, and general paresis (dementia paralytica)2). In particular, a gumma is a form of granuloma16). The presence of necrosis and plasma cells differentiates gummas from sarcoidosis2). But, tertiary syphilis containing few plasma cells and necrotizing sarcoid granulomas are occasionally reported14,19,21).
In literature review, there are several reports of cases of secondary or tertiary syphilis mimicking sarcoidosis including non-intracranial lesion3,7,13,15). Although there are various diseases included in differential diagnosis of neurosarcoidosis, neurosyphilis should be included in a differential diagnosis of neurosarcoidosis, particularly in patients with a history of syphilis.
References
1. Cain H, Kraus B. Asteroid bodies : derivatives of the cytosphere. An electron microscopic contribution to the pathology of the cytocentre. Virchows Arch B Cell Pathol. 1977; 26:119–132. PMID: 204105.
2. Carlson JA, Dabiri G, Cribier B, Sell S. The immunopathobiology of syphilis : the manifestations and course of syphilis are determined by the level of delayed-type hypersensitivity. Am J Dermatopathol. 2011; 33:433–460. PMID: 21694502.

3. Edmonds LC, Stubbs SE, Ryu JH. Syphilis : a disease to exclude in diagnosing sarcoidosis. Mayo Clin Proc. 1992; 67:37–41. PMID: 1732690.
4. Elias WJ, Lanzino G, Reitmeyer M, Jane JA. Solitary sarcoid granuloma of the cerebellopontine angle : a case report. Surg Neurol. 1999; 51:185–190. PMID: 10029426.
5. Gascón-Bayarri J, Mañá J, Martínez-Yélamos S, Murillo O, Reñé R, Rubio F. Neurosarcoidosis : report of 30 cases and a literature survey. Eur J Intern Med. 2011; 22:e125–e132. PMID: 22075297.
6. Harding AS, Ghanem KG. The performance of cerebrospinal fluid treponemal-specific antibody tests in neurosyphilis : a systematic review. Sex Transm Dis. 2012; 39:291–297. PMID: 22421696.

7. Hervier B, Wastiaux H, Freour T, Masseau A, Corvec S, Armingeat T, et al. [Sarcoidosis-like granulomatosis revealing a tertiary syphilis]. Rev Med Interne. 2009; 30:806–808. PMID: 19249139.
8. Hoitsma E, Faber CG, Drent M, Sharma OP. Neurosarcoidosis : a clinical dilemma. Lancet Neurol. 2004; 3:397–407. PMID: 15207796.
10. Khoury J, Wellik KE, Demaerschalk BM, Wingerchuk DM. Cerebrospinal fluid angiotensin-converting enzyme for diagnosis of central nervous system sarcoidosis. Neurologist. 2009; 15:108–111. PMID: 19276791.

11. Kumar G, Kang CA, Giannini C. Neurosarcoidosis presenting as a cerebellar mass. J Gen Intern Med. 2007; 22:1373–1376. PMID: 17619108.

12. Marra CM, Tantalo LC, Maxwell CL, Ho EL, Sahi SK, Jones T. The rapid plasma reagin test cannot replace the venereal disease research laboratory test for neurosyphilis diagnosis. Sex Transm Dis. 2012; 39:453–457. PMID: 22592831.

13. Perry HO, Lofgren RK. Secondary and tertiary syphilis presenting as sarcoidal reactions of the skin. Cutis. 1984; 34:253–255. 257–258. PMID: 6488885.
14. Rolfes DB, Weiss MA, Sanders MA. Necrotizing sarcoid granulomatosis with suppurative features. Am J Clin Pathol. 1984; 82:602–607. PMID: 6093497.

15. Singh R, Kaur D, Parameswaran M. Sarcoidal reaction of the skin in syphilis. Br J Vener Dis. 1971; 47:209–211. PMID: 5090748.

16. Spector WG, Heesom N. The production of granulomata by antigen-antibody complexes. J Pathol. 1969; 98:31–39. PMID: 4900707.

17. Spencer TS, Campellone JV, Maldonado I, Huang N, Usmani Q, Reginato AJ. Clinical and magnetic resonance imaging manifestations of neurosarcoidosis. Semin Arthritis Rheum. 2005; 34:649–661. PMID: 15692958.

18. Stübgen JP. Neurosarcoidosis presenting as a retroclival mass. Surg Neurol. 1995; 43:85–87. discussion 87-88. PMID: 7701433.

19. Tanabe JL, Huntley AC. Granulomatous tertiary syphilis. J Am Acad Dermatol. 1986; (2 Pt 2):15:341–344. PMID: 3734178.

20. Terushkin V, Stern BJ, Judson MA, Hagiwara M, Pramanik B, Sanchez M, et al. Neurosarcoidosis: presentations and management. Neurologist. 2010; 16:2–15. PMID: 20065791.
21. Tobias S, Prayson RA, Lee JH. Necrotizing neurosarcoidosis of the cranial base resembling an en plaque sphenoid wing meningioma : case report. Neurosurgery. 2002; 51:1290–1294. discussion 1294. PMID: 12383376.

23. Zajicek JP, Scolding NJ, Foster O, Rovaris M, Evanson J, Moseley IF, et al. Central nervous system sarcoidosis--diagnosis and management. QJM. 1999; 92:103–117. PMID: 10209662.

Fig. 1
Gadolinium-enhanced magnetic resonance (MR) images. A : Preoperative MR image revealing the mass extended toward the middle fossa laterally and to the sphenoid sinus anteriorly. This lesion also extended to the cerebellopontine angle posteriorly with a compression of the brain stem. B : Postoperative MR image revealing resolution of the mass over the cavernous sinus region sinus.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download