Abstract
A correlation between radiation therapy and cavernoma has been suspected since 1994. Since then, only a few cases of radio-induced cavernomas have been reported in the literature (85 patients). Most of them were children, and the most frequent original tumour had been medulloblastoma. The authors report a case of two cystic cavernous angiomas after radiation therapy for atypical meningioma in adult woman. This is the first case of cavernous angioma after radiotherapy for low grade meningioma. A 39-year-old, Latin american woman was operated on for a frontal atypical meningioma with intradiploic component and adjuvant radiotherapy was delivered (6000 cGy local brain irradiation, fractionated over 6 weeks). Follow-up MR imaging showed no recurrences of the tumour and no other lesions. Ten years later, at the age of 49, she consulted for progressive drug-resistant headache. MR imaging revealed two new well defined areas of different signal intensity at the surface of each frontal pole. Both lesions were surgically removed; the histopathological diagnosis was cavernous angioma. This is the first case of cavernous angioma after radiation therapy for atypical meningioma : it confirms the development of these lesions after standard radiation therapy also in patients previously affected by non-malignant tumours.
Cavernous angioma (CA), also known as cavernous hemangioma, cavernoma, cavernous malformation, is the most common slow-flow vascular malformation. CAs are familial in 30-50% of cases, and they were considered to be congenital lesions for a long time13). However, "de novo" cases are now well documented8), either in familial or sporadic forms, and it seems likely that mechanisms for development of acquired lesions exist. A correlation between radiation therapy and cavernoma has been suspected since 19943). Since then, additional cases have appeared in the literature and most cases have been in children.
In this paper, the authors describe the case of an adult patient who presented two radiation-induced cavernous angiomas after receiving radiation therapy for atypical meningioma. This is the first case of cavernous angioma after radiation therapy for low grade meningioma.
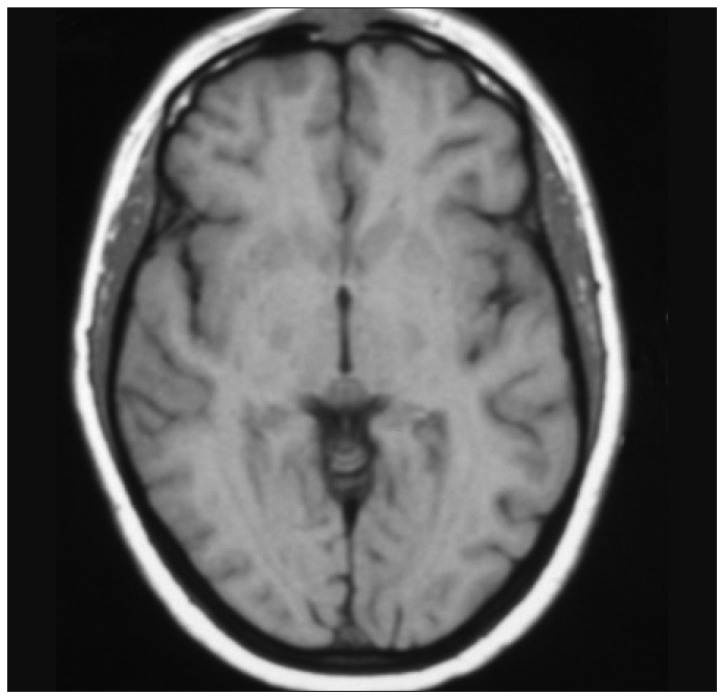
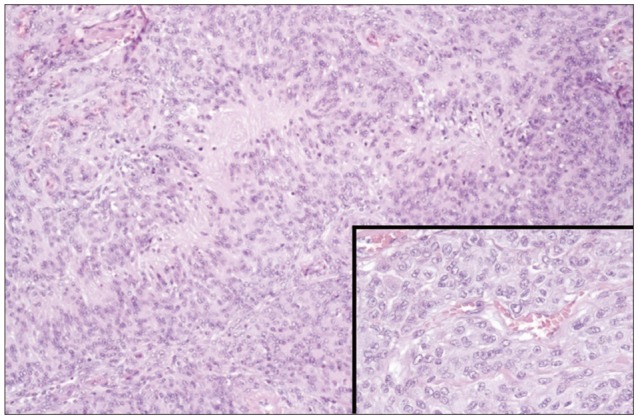
A 49-year-old, Latin American, right handed, woman, at the age of 39 consulted for progressive swelling of the left frontal region. Magnetic Resonance (MR) scanning showed an intradiploic mass with dural attachment (Fig. 1). A Simpson I removal was achieved at the first operation; on histological examination (Fig. 2) the diagnosis was atypical meningioma (World Health Organization II).
The patient received 6000 cGy of Fractionated Stereotactic Radiotherapy. Chemotherapy was not delivered. The patient was followed up neurologically and radiologically. Follow-up MR imaging did not reveal any recurrence of the tumour or other lesions.
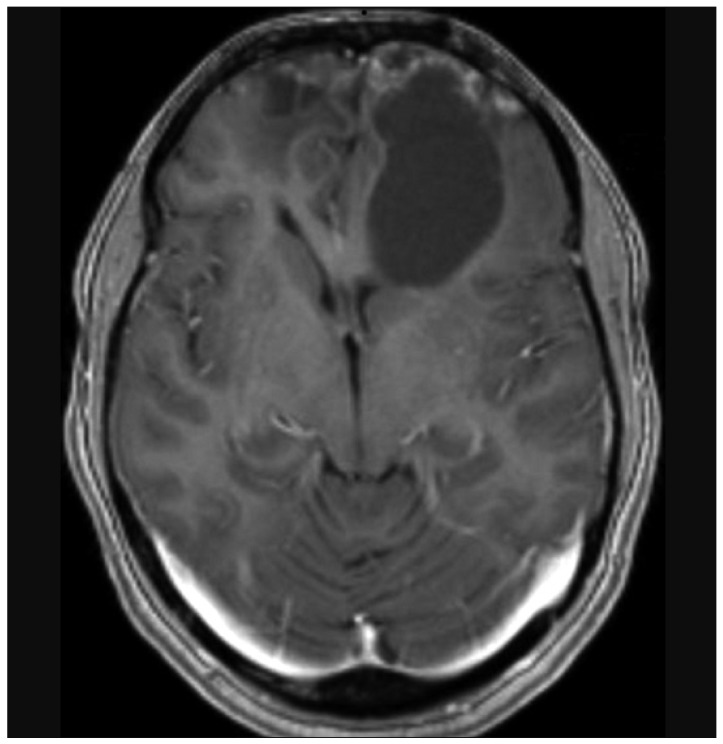
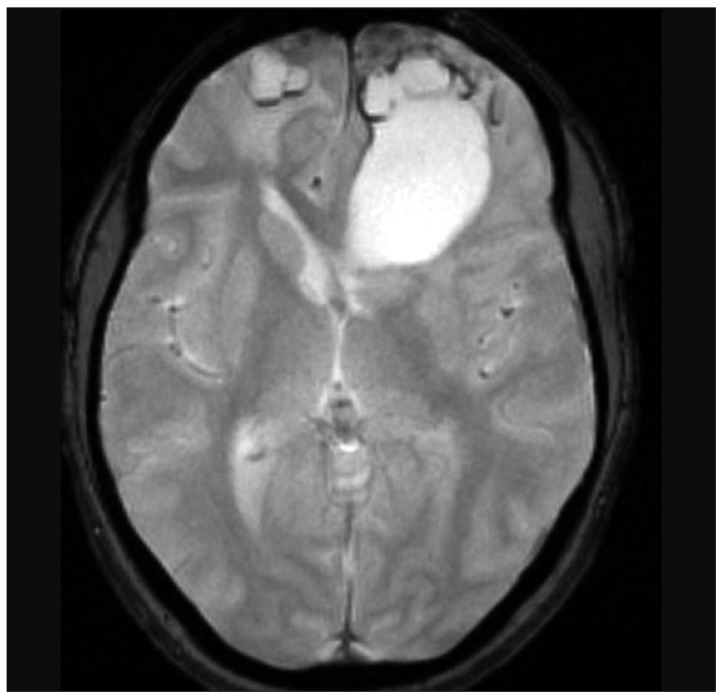
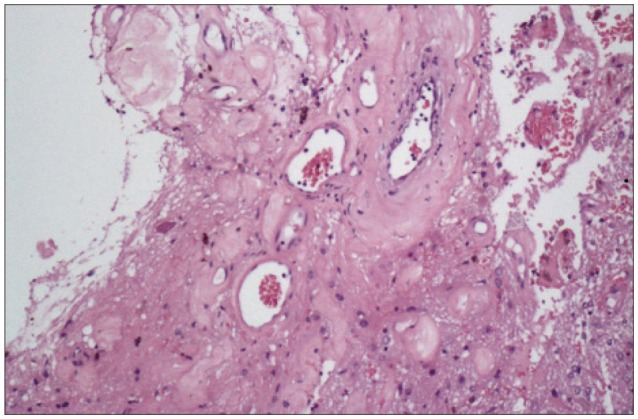
Nine years later, at the age of 48, follow-up MR imaging showed two new well defined areas, with cystic and necrotic components, at the surface of each frontal pole. The lesions appeared isointense-hypointense on T1-weighted sequences, with heterogeneous gadolinium enhancement, and hyperintense on T2-weighted. Clinical examination was normal. The following year, at the age of 49, she presented to the emergency unit of our department with drug-resistant headache. MR imaging showed that both lesions had grown. The largest one (5.5×3.6 cm), in the left frontal lobe, was a large gliotic bordered lobulated lesion, with a big cystic component, that extended from the frontal pole to the frontal horn of lateral ventricle. The right lesion (3.7×2.4 cm), with a smaller cystic component, was entirely within the right pole (Fig. 3, 4). Bilateral frontal craniotomy was performed to remove both the masses. The histopathological diagnosis was cavernous angioma (Fig. 5). The lesion was composed of hyalinized vascular channels, with different calibers. The vessels were thick and amuscular and the brain parenchyma interposed between the channels showed gliosis. The patient had not family history of cavernomas. After surgical removal, symptomatology completely remitted.
Cerebral cavernous angiomas are vascular malformation composed of back-to-back hyalinised vascular channels. Brain parenchyma can be interposed among the channels14), features evident in our cases where reactive gliosis has been observed. On histological examination are evident loosely apposed, engorged vessels with hyalinized vascular vessels. Secondary alterations such as thrombosis are common, as in our lesions, where lumens are occluded by thrombi. Neighbouring brain parenchyma shows a gliotic aspect, with haemorrhagic extravasations and hemosiderin6). There are no obvious histopathological differences between congenital and acquired lesions. In our case, we posed a diagnosis of radio-induced cavernous angioma based on MR images : it did not show any malformations, even in GRE-T2-weighted sequences at the time of the first operation.
Only a few cases of cavernomas induced by radiation treatment, 85 patients, have been reported in the literature1,2,4,5,9,11). Of these, 35 were female and 47 were male (the sex was not reported in three patients).
There are only hypotheses to explain why these lesions occur. Some authors suggested that the ionizing effect of radiation energy generates vascular and connective tissue changes in the stroma12). Others proposed it is possible that cavernomas are present before radiation therapy but are radiographically occult at the time of treatment, and it is only with radiation exposure that they grew and became visible on MR. Most cases have been children and young patients. The mean age of the patients was 11.7 years9); only 7 patients (8.2%) were older than 40 years. Most patients were irradiated at ≤10 years of age (56, 65.9%); only four were irradiated at ≥30 years of age (4.7%)9,11). It is still unclear why cavernous malformations appeared at a higher rate in children after radiation therapy than in adults2,7). Some authors have illustrated that the immature brain of the paediatric patient is more sensitive to radiation than the adult brain and radiation therapy can result in vessel wall necrosis, dilation of the vessel lumen, hyalinization, fibrosis, and mineralization that predisposes to cavernoma formation.
Radio-induced cavernomas may occur even after many years from the radiation. Reported latency periods range from 1 to 26 years9), mean is 10.3±1.9.
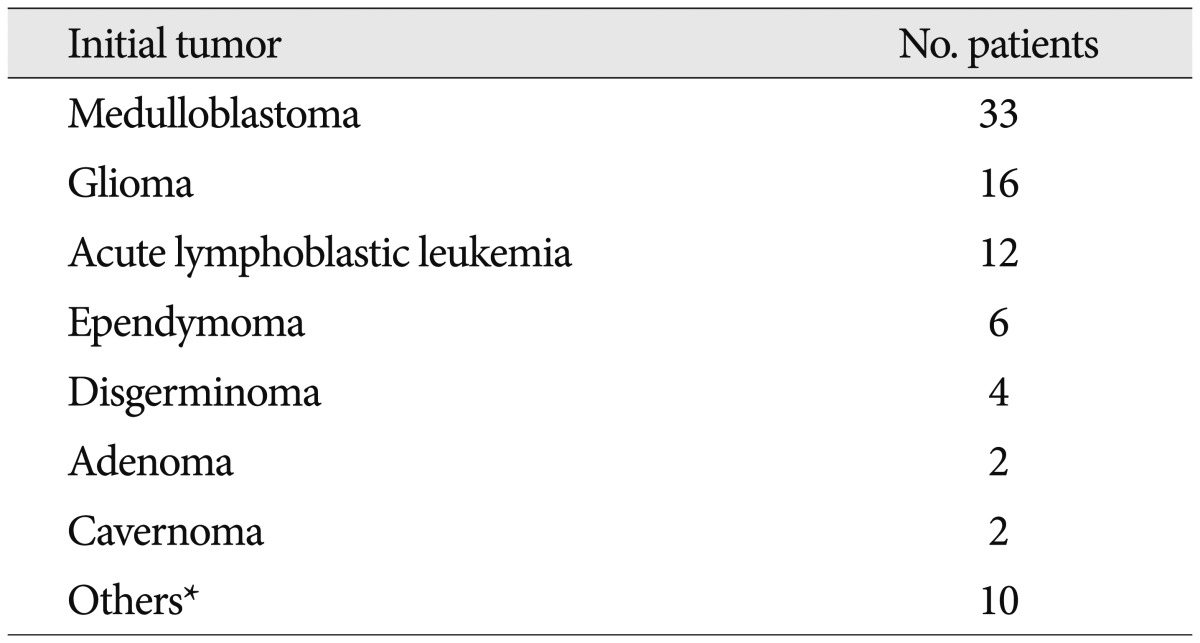
The initial tumours are summarized in Table 1. No case of cavernous angioma after radiotherapy for low grade meningiomas was reported. This is the first case of cavernous angioma after radiation therapy for atypical meningioma.
Two or more cavernous angiomas were found in 41% of patients. There were no significant relationships among the development of multiple cavernous angioma and radiation dose, age at irradiation, or latency to diagnosis9). Moriarity et al.10), in his prospective series of 68 patients, found multiple lesions were more likely in patients with familial disease than in patients with sporadic disease (85% vs. 25%).
Cavernous hemangiomas are rare but important sequelae of radiotherapy. To fully elucidate why radiation-induced cavernous malformations are rare in adults, it will be necessary to collect more cases. This is the first case of cavernous angioma after radiation therapy for atypical meningioma : it confirms the development of these lesions after standard radiation therapy also in patients previously affected by non-malignant tumours.
References
1. Battaglia F, Uro-Coste E, Delisle MB, Tannier C. [Radiation-induced cavernoma : two cases]. Rev Neurol (Paris). 2008; 164:468–471. PMID: 18555880.
2. Burn S, Gunny R, Phipps K, Gaze M, Hayward R. Incidence of cavernoma development in children after radiotherapy for brain tumors. J Neurosurg. 2007; 106(5 Suppl):379–383. PMID: 17566205.

3. Ciricillo SF, Cogen PH, Edwards MS. Pediatric cryptic vascular malformations : presentation, diagnosis and treatment. Pediatr Neurosurg. 1994; 20:137–147. PMID: 8161487.

4. Duhem R, Vinchon M, Leblond P, Soto-Ares G, Dhellemmes P. Cavernous malformations after cerebral irradiation during childhood : report of nine cases. Childs Nerv Syst. 2005; 21:922–925. PMID: 15662523.

5. Furuse M, Miyatake SI, Kuroiwa T. Cavernous malformation after radiation therapy for astrocytoma in adult patients : report of 2 cases. Acta Neurochir (Wien). 2005; 147:1097–1101. discussion 1101. PMID: 16021386.

6. Günel M, Awad IA, Finberg K, Steinberg GK, Craig HD, Cepeda O, et al. Genetic heterogeneity of inherited cerebral cavernous malformation. Neurosurgery. 1996; 38:1265–1271. PMID: 8727164.

7. Heckl S, Aschoff A, Kunze S. Radiation-induced cavernous hemangiomas of the brain : a late effect predominantly in children. Cancer. 2002; 94:3285–3291. PMID: 12115362.

8. Jain R, Robertson PL, Gandhi D, Gujar SK, Muraszko KM, Gebarski S. Radiation-induced cavernomas of the brain. AJNR Am J Neuroradiol. 2005; 26:1158–1162. PMID: 15891176.
9. Keezer MR, Del Maestro R. Radiation-induced cavernous hemangiomas : case report and literature review. Can J Neurol Sci. 2009; 36:303–310. PMID: 19534329.

10. Moriarity JL, Wetzel M, Clatterbuck RE, Javedan S, Sheppard JM, Hoenig-Rigamonti K, et al. The natural history of cavernous malformations : a prospective study of 68 patients. Neurosurgery. 1999; 44:1166–1171. discussion 1172-1173. PMID: 10371615.

11. Nimjee SM, Powers CJ, Bulsara KR. Review of the literature on de novo formation of cavernous malformations of the central nervous system after radiation therapy. Neurosurg Focus. 2006; 21:e4. PMID: 16859257.

12. Nyáry I, Major O, Hanzély Z, Szeifert GT. Histopathological findings in a surgically resected thalamic cavernous hemangioma 1 year after 40-Gy irradiation. J Neurosurg. 2005; 102(Suppl):56–58. PMID: 15662782.

13. Rigamonti D, Hadley MN, Drayer BP, Johnson PC, Hoenig-Rigamonti K, Knight JT, et al. Cerebral cavernous malformations. Incidence and familial occurrence. N Engl J Med. 1988; 319:343–347. PMID: 3393196.
14. Tomlinson FH, Houser OW, Scheithauer BW, Sundt TM Jr, Okazaki H, Parisi JE. Angiographically occult vascular malformations : a correlative study of features on magnetic resonance imaging and histological examination. Neurosurgery. 1994; 34:792–799. discussion 799-800. PMID: 8052376.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download