Abstract
A case of intractable hiccup developed by cavernous hemangioma in the medulla oblongata is reported. There have been only five previously reported cases of medullary cavernoma that triggered intractable hiccup. The patient was a 28-year-old man who was presented with intractable hiccup for 15 days. It developed suddenly, then aggravated progressively and did not respond to any types of medication. On magnetic resonance images, a well-demarcated and non-enhancing mass with hemorrhagic changes was noted in the left medulla oblongata. Intraoperative findings showed that the lesion was fully embedded within the brain stem and pathology confirmed the diagnosis of cavernous hemangioma. The hiccup resolved completely after the operation. Based on the presumption that the medullary cavernoma may trigger intractable hiccup by displacing or compression the hiccup arc of the dorsolateral medulla, surgical excision can eliminate the symptoms, even in the case totally buried in brainstem.
Hiccup is a repeated involuntary, spasmodic, and temporary contraction of the diaphragm accompanied by a sudden closure of the glottis, producing the characteristic inspiratory sound "hic" and discomfort9). It can be considered persistent or intractable when it lasts more than 24 hours11). The exact etiology of hiccup remains unclear in most cases. Regarding various central causes, the medulla oblongata has been investigated as one of the most important centers in the hiccup circuit. In addition to neurological disorders, including medullary infarction and hemorrhage, tumors and tuberculoma reportedly generate intractable hiccup2,7,17).
Although few reports demonstrated surgically treated and pathologically confirmed cavernous hemangioma (CH) in the medulla oblongata4,11,12,15,18), most of them were superficially located in the dorsal part of the medulla oblongata. In the current case, the authors demonstrate the fully embedded medullary cavernoma with intractable hiccup surgically treated without morbidity and discuss possible pathogenesis of this condition with a review of the reported cases and related literature.
A 28-year-old man presenting with persistent hiccup for 15 days was admitted to our hospital. The symptom developed suddenly and aggravated progressively in its frequency and intensity. The patient noticed motor weakness and sensory changes in the left side of his body three days prior to admission. Hiccup did not respond to any types of medication, but occurred only occasionally while asleep. There were no abnormal findings in endoscopy and computed tomography scans for the chest and abdominal organs.
On admission, neurological examination revealed no impairment of mental status, definitive sensory or motor deficits, gaits difficulties, or abnormal deep tendon reflex. The cranial nerves were also unaffected. Magnetic resonance (MR) imaging showed a 1.5×1.5×2 cm nodule in the left medulla oblongata with peripheral hyperintensity on T1-weighted images and predominantly low signal intensity on T2-weighted images (Fig. 1). The lesion was not enhanced by administration of gadolinium. Vertebral angiography demonstrated no staining of the lesion.
On presumptive diagnosis of cavernous hemangioma in the medulla oblongata, surgery was performed via the midline suboccipital approach under prone position. The lesion was covered by normal parenchymal tissue and made a bulging contour of the medulla oblongata with superficial abnormal draining veins (Fig. 2A). The shortest trajectory to the lesion was confirmed by neuronavigation (StealthStation S7®, Medtronic, Minneapolis, MN, USA) and a 2 cm longitudinal pial incision was made caudally from the obex. After dissecting a few millimeters deep, hemosiderin staining of the neural tissue was encountered (Fig. 2B). The plane of the dissection between the cavernoma and the parenchyma was well-distinguished (Fig. 2C). The lesion was removed en bloc. Histopathologically, the brain specimen revealed irregularly dilated vascular spaces without intervening neural tissue, which are typical features of cavernous hemangioma (Fig. 3). Additionally, there was reactive piloid gliosis with numerous Rosenthal fibers in the periphery of the lesion and occasional hemosiderin-laden macrophages. Hiccup resolved immediately after surgery. The patient had slight hemiparesis (motor grade IV+/IV+) and hemisensory changes that cleared entirely at the time of discharge.
The clinical manifestations of the brainstem CHs closely correlated with the anatomical location of the lesion. The common signs and symptoms include various types of cranial neuropathy, sensory/motor deficits, headache, diplopia, ataxia, vertigo, nausea/vomiting, dysarthria, dysphagia, and dysmetria, but hiccup has rarely been reported1). Porter et al.16), in their review of 100 cases of brainstem cavernous malformations, reported hiccup as the presenting symptom in three cases. Contrary to this, Ward et al.20) reported that intractable hiccup was not infrequent clinical presentation in medullary cavernoma (5/18 cases, 27.8%). However, these case series did not reveal the detail description on the exact location, and clinico-radiological characteristics. For this reason, medullary caverrnoma presenting as intractable hiccup has been reported as a single case report, with the explanation of unique clinical course (Table 1)4,11,12,15,18). Majority of reported cases were in male patients (female in only one case) with the mean age of 34.8 years, relatively younger than the patients with general brainstem CHs in a large series (41.8 years in brainstem CHs; 44.1 years in medullary CH)1). Main accompanying symptoms were sensory/motor deficits, followed by visual disturbance, headache or nausea and vomiting. Intralesional hemorrhage, related with sudden onset natures of hiccup, occurred in all cases and the size of the lesion was varied (range from smaller than 1 cm up to 2.2 cm). Medullary CHs tend to be the smallest among brainstem CHs (mean diameter, 1.2 cm in medullary CHs vs. 1.8 cm in total brainstem CHs), while the preoperative hemorrhagic rate approaches 97% in the medullary CHs and in brainstem CHs as well1). The hiccup was immediately resolved after the surgical resection in all cases.
With regard to the pathomechanism of hiccup, Hassler assumed that hiccup could be the subcortical equivalent of myoclonus generated at the pontomedullary level of the triangle of Guillan-Mollaret (inferior olivary nucleus, dentate nucleus, and red nucleus)5). Other investigators proposed that hiccup could be resultant from denervation supersensitivity caused by dysfunction of the inferior olivary complex, nucleus ambiggus and adjacent reticular formation of medullar oblongata8,10,19). In animal studies, hiccup-like responses were generated by electrical stimulation in the medullary reticular formation, lateral to the nucleus ambiggus and rostral to the obex3). Oshima et al.13) also showed that GABA-containing inhibitory cells in the nucleus raphe magnus could be the source of inhibitory inputs to the hiccup reflex arc.
Medullary lesions other than medullary CHs also reportedly induced hiccups in the dorsolateral aspect of the medullar oblongata6,7,21). In a study conducted by Park et al.14), 14% of patients with lateral medullary infarcts (seven out of 51) had hiccups, mainly when the lesion developed in the dorsolateral region of the middle medulla. All reported cases of medullary CHs, inducing intractable hiccup (except one case with ill-defined location), demonstrated laterally-located lesions in the medulla oblongata; three were superficial exophytic cavernomas and the other two, totally buried into medulla. In addition, the intractable hiccup in the reports resolved immediately after surgery. The location of the former three exophytic cases seems to be in the dorsolateral medulla rostral to the obex, described by Arita et al.3), in which the pathophysiology may be related to excitatory function for hiccup genesis. On the other hand, the latter two cases, including the current one, were located in the deep portion of the dorsolateral medulla caudal to the obex. Intractable hiccup in the latter ones may be induced by inactivation of the inhibitory function of GABA-containg neurons suggested by Oshima et al.13) Considering the aforementioned hiccup pathogenesis and the case summary, we presume that the present medullary cavernoma may also have reduced the inhibitory function or induced a stimulatory signal on the hiccup reflex by displacing or compressing the hiccup arc of the dorsolateral medulla.
References
1. Abla AA, Lekovic GP, Turner JD, de Oliveira JG, Porter R, Spetzler RF. Advances in the treatment and outcome of brainstem cavernous malformation surgery : a single-center case series of 300 surgically treated patients. Neurosurgery. 2011; 68:403–414. discussion 414-415. PMID: 21654575.
2. al Deeb SM, Sharif H, al Moutaery K, Biary N. Intractable hiccup induced by brainstem lesion. J Neurol Sci. 1991; 103:144–150. PMID: 1880531.

3. Arita H, Oshima T, Kita I, Sakamoto M. Generation of hiccup by electrical stimulation in medulla of cats. Neurosci Lett. 1994; 175:67–70. PMID: 7970214.

4. Eisenächer A, Spiske J. Persistent hiccups (singultus) as the presenting symptom of medullary cavernoma. Dtsch Arztebl Int. 2011; 108:822–826. PMID: 22211149.

5. Hassler R. Die neuronalen system der extrapyramidalen myoclonien und deren stereotaktische behandlung. In : Doose H, editor. Aktuelle Neuropadiatrie. Stuttgart: Thieme;1997. p. 20–46.
6. Johnson DL. Intractable hiccups : treatment by microvascular decompression of the vagus nerve. Case Report. J Neurosurg. 1993; 78:813–816. PMID: 8468612.

7. Kumral E, Acarer A. Primary medullary haemorrhage with intractable hiccup. J Neurol. 1998; 245:620–622. PMID: 9758303.

8. Lapresle J, Hamida MB. The dentato-olivary pathway. Somatotopic relationship between the dentate nucleus and the contralateral inferior olive. Arch Neurol. 1970; 22:135–143. PMID: 4188259.
9. Launois S, Bizec JL, Whitelaw WA, Cabane J, Derenne JP. Hiccup in adults : an overview. Eur Respir J. 1993; 6:563–575. PMID: 8491309.
10. Matsuo F, Ajax ET. Palatal myoclonus and denervation supersensitivity in the central nervous system. Ann Neurol. 1979; 5:72–78. PMID: 34357.

11. Mattana M, Mattana PR, Roxo MR. Intractable hiccup induced by cavernous angioma in the medulla oblongata : case report. J Neurol Neurosurg Psychiatry. 2010; 81:353–354. PMID: 20185478.

12. Musumeci A, Cristofori L, Bricolo A. Persistent hiccup as presenting symptom in medulla oblongata cavernoma : a case report and review of the literature. Clin Neurol Neurosurg. 2000; 102:13–17. PMID: 10717396.

13. Oshima T, Sakamoto M, Tatsuta H, Arita H. GABAergic inhibition of hiccup-like reflex induced by electrical stimulation in medulla of cats. Neurosci Res. 1998; 30:287–293. PMID: 9678632.

14. Park MH, Kim BJ, Koh SB, Park MK, Park KW, Lee DH. Lesional location of lateral medullary infarction presenting hiccups (singultus). J Neurol Neurosurg Psychiatry. 2005; 76:95–98. PMID: 15608002.

15. Pechlivanis I, Seiz M, Barth M, Schmieder K. A healthy man with intractable hiccups. J Clin Neurosci. 2010; 17:781–783. PMID: 20359895.

16. Porter RW, Detwiler PW, Spetzler RF, Lawton MT, Baskin JJ, Derksen PT, et al. Cavernous malformations of the brainstem : experience with 100 patients. J Neurosurg. 1999; 90:50–58. PMID: 10413155.
17. Stotka VL, Barcay SJ, Bell HS, Clare FB. Intractable hiccough as the primary manifestation of brain stem tumor. Am J Med. 1962; 32:312–315. PMID: 13917547.

18. Thaci B, Burns JD, Delalle I, Vu T, Davies KG. Intractable hiccups resolved after resection of a cavernous malformation of the medulla oblongata. Clin Neurol Neurosurg. 2013; 115:2247–2250. PMID: 23932467.

19. Turazzi S, Alexandre A, Bricolo A, Rizzuto N. Opsoclonus and palatal myoclonus during prolonged post-traumatic coma. A clinico-pathologic study. Eur Neurol. 1977; 15:257–263. PMID: 913438.

20. Wang CC, Liu A, Zhang JT, Sun B, Zhao YL. Surgical management of brain-stem cavernous malformations : report of 137 cases. Surg Neurol. 2003; 59:444–454. discussion 454. PMID: 12826334.

21. Ward BA, Smith RR. Hiccups and brainstem compression. J Neuroimaging. 1994; 4:164–165. PMID: 8061384.

Fig. 1
Preoperative MRI. A : Axial T2-weighted image shows a nodular lesion in the left sided cervicomedullary junction, with marked hyposignal intensity containing internal foci of hypersignal intensity. Sagittal pre- (B) and post- (C) gadolinium T1WI images show a non-enhancing cavernous hemangioma surrounded by bright hypersignal intensity halo which is consistent with blood products. D : An associated small developmental venous anomaly (arrow) is visualized in superior aspect of the cavernous hemangioma on axial post-gadolinium T1WI image.
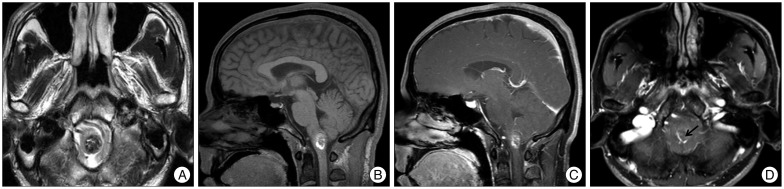
Fig. 2
Peroperative photographs (A) demonstrating slightly bulged medulla oblongata with superficial abnormal draining vein (arrow). Note that the mass was located caudally to obex and removed using hemosiderin plane between hemangioma and normal parenchyme (B and C). *: obex.
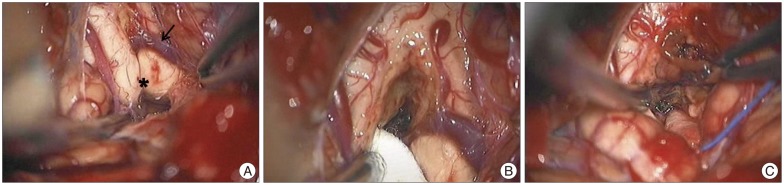
Fig. 3
Microscopic findings of the medullary cavernous hemangioma. Histopathologic exam reveals a typical cavernous hemangioma with irregularly dilated vascular spaces. The vascular walls are accompanied by cautery artifacts (hematoxylin-eosin, original magnification, ×200).
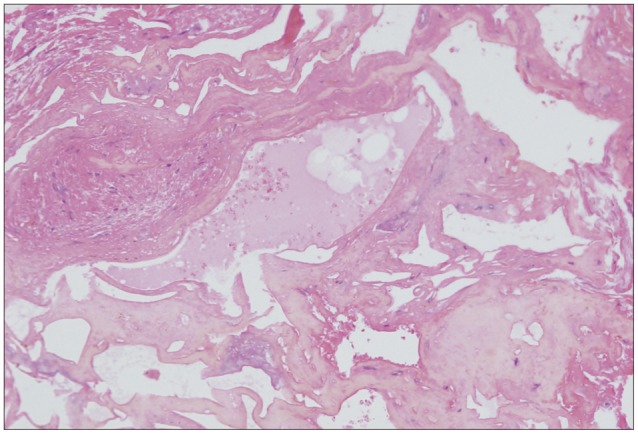
Table 1
Surgically resected medullary cavernomas presenting as intractable hiccup
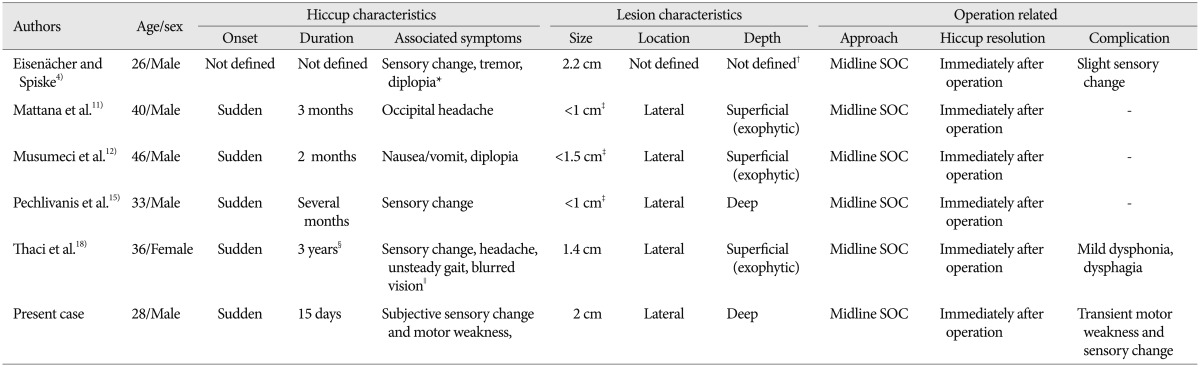
*Presenting sudden deterioration in consciousness and respiration due to intralesional hemorrhage, †May be deep seated lesion based on representative MR images, ‡Presumptive size of lesion based on representative MR images, §Recurrent and lasting up to 3 months, ∥Presenting sudden developed quadriparesis, headache, unsteady gait, and facial paresis due to intralesional hemorrhage. SOC : suboccipital craniotomy




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download