Abstract
Computed tomography angiography (CTA) is commonly used in setting of subarachnoid hemorrhage, but imaging features of aneurysm rupturing taking place at the time of scanning has rarely been described. The author reports a case of actively rebleeding aneurysm of the anterior communicating artery with intraventricular extravasation on the hyperacute CTA imaging. The rebleeding route, not into the third ventricle but into the lateral ventricles, can be visualized by real-time three-dimensional CT pictures. The hemorrhage broke the septum pellucidum and the lamina rostralis rather than the lamina terminalis.
Three-dimensional (3D) computed tomography angiography (CTA) is now utilized as the first imaging modality for detecting underlying lesions in patients with acute subarachnoid hemorrhage (SAH)1). In spite of its increasing application, the reports of bleeding from aneurysms during CTA examination are few, so little information is available regarding the imaging findings for extravasation of ruptured cerebral aneurysms8,18). Furthermore, intraventricular hemorrhage (IVH) is commonly developed during rupture of anterior communicating artery (ACoA) aneurysm; however the CT picture for anatomical pathway of this condition has not been demonstrated2). The author describes a case of rerupturing ACoA aneurysm in which the blood is spilled through the lamina rostralis and the septum pellucidum into the ventricular system during CTA performed in the acute stage.
A 52-year-old woman with hypertension presented to the emergency room within 50 minutes after developing severe headache and lethargic mentality. Her blood pressure was 160/100 mm Hg on arrival; the infusion of nicardipine hydrochloride reduced it to below 130 mm Hg before CT scanning. An emergent unenhanced CT scan (SOMATOM Definition Flash, Siemens, Forchheim, Germany) showed diffuse SAH and subdural hematoma (Fig. 1A). The CTA was then performed to assess for an underlying aneurysm. To enhance the cerebral arteries, 120 mL nonionic contrast media was infused into the antecubital vein at a flow rate of 4 mL/s using a power injector. The delay between the infusion of contrast and the start of scanning was 40 seconds. The CTA source images were reformatted with the volume rendering method using a workstation (Wizard, Siemens, Forchheim, Germany). Aneurysms at ACoA and middle cerebral artery were demonstrated on the infused CT scan done 1.5 hours after admission (Fig. 1B). This patient suddenly fell into a deep stupor immediately after the CTA examination. Postcontrast CT scans taken after CTA revealed enlargement of SAH and new IVH in the lateral ventricle (Fig. 1C, D).
The results of brain CTA clearly visualized an ACoA aneurysm from which the contrast medium was actively ejecting. An area consistent with contrast jet was identified around the aneurysm (Fig. 2A). The maximum intensity projection illustrated the diffuse curvilinear spill out of the contrast medium arising from the aneurysm dome, extended into the lateral ventricle (Fig. 2B). The author realized that this vessel-like extravasation represented the aneurysm rebleeding and the consequential contrast swirling at the time of imaging. The extravasations of blood were detected in the preexisting intracerebral hematomas on the raw data of the CTA, and they had the same high densities as the internal carotid artery (Fig. 3A-D). On sagittal reformed 3D CT images, the clots developed from the first aneurysm bleeding clearly delineated the interhemispheric cistern, the lamina terminalis, and the corpus callosum, similar to a ventriculographic manner. The leaking trajectory of contrast medium, ejected from ACoA aneurysm, was 1) through the lamina rostralis above the lamina terminalis, 2) into the space between the septal leaves and 3) into the frontal horn of the lateral ventricle (Fig. 3E, F). The coronal CT scans dynamically depicted the hemorrhage from ACoA aneurysm broke through the initial intraseptal hematoma and extended into the left frontal horn (Fig. 3G, H).
The authors were certain that the aneurysm rerupture occurred while the routine CT scans for 3D angiography were being acquired. The patient was intubated for declining mental status and was urgently transferred to the operating room. The aneurysms were clipped simultaneously after hematoma evacuation. The surgeon did not find perforation of the lamina terminalis from direct hemorrhage of ACoA aneurysm. She regained consciousness and was discharged 3 weeks later after the event. This patient recovered neurologically including most of her cognitive functions, although with minimal memory deficit persisting at the 5-year follow-up.
Recently, brain CTA can be immediately performed after the recognition of SAH on precontrast CT in many institutions. This expedites an early triage to the proper management and avoids delay between the diagnostic angiogram and early aneurysm repair24). Nevertheless, rebleeding from ruptured aneurysms is a still major cause of morbidity and death in the patients presenting with spontaneous SAH13).
Rupture of aneurysms or other vascular malformations during conventional cerebral angiography has been frequently reported previously19). The published cases may reflect iatrogenic aneurysm rupture from forceful contrast infusion into the carotid artery10,21). Such consideration does not apply to CTA, where the contrast agent is administered slowly into a peripheral vein. There have been few studies in which the causal relationship between performance of CT scan and rupture of the aneurysm has been discussed2,5,14). For this reason, the frequency of aneurysm rupture during CTA has not been defined, but it is seems to be extremely rare. In the case reported here, the CTA showing active contrast leakage was performed within 3 hours of initial aneurysm rupture. Accordingly, it is likely that the cause of this bleeding from ACoA aneurysm was due to a second spontaneous hemorrhage, which coincided with CT scanning.
Besides a spontaneous bleeding, direct effects of contrast material are supplementary factors for hemorrhage in reported ruptured aneurysms during CTA10,25). The previously documented potentiality was that injuring endothelium and activating anticoagulant could initiate rebleeding from an aneurysm which is only coated by a platelet plug at an acute phase of SAHs11,12). Moreover, some patients obviously are intolerable to contrast medium very well, as they usually vomited after injection of 30 mL (the total dose given was up to 200 mL)6,16). An increased blood pressure during emesis might be incriminated as a cause for intra-CTA rebleedings. In addition, the physical activity necessary for the examination procedure might be considered casual as well20). Therefore, more attention should be paid to deep sedation and strict blood pressure control before and during CTA evaluation especially for at-risk patients of early rebleeding3,17). Practically, an antifibrinolytic therapy might be helpful for reducing the aneurysm rebleeding for the patients immediately transferred after the onset like the author's case22).
As the rebleeding of aneurysm resuls in catastrophic consequences, it is imperative to understand the specific features of continuous bleeding on the CTA images. On 3D reformatted images of the current case, the contrast extravasation was depicted to have a ribbon-like appearance that mimicked a vascular structure, and it had a long trajectory traveling through the subarachnoid space, the preexistent hematoma, and the ventricles. Initially, it was misconstrued as a movement artifact, but soon after, it was correctly interpreted as an aneurysm bursting. The prior reports have described other patterns of a blood extravasation; cap sign, corkscrew sign, hematoma opacification sign, starburst appearance, and nebulous high attenuation4,5,6,16). This variability in imaging features seemed to depend on the following factors; site of rupture of the aneurysm, the location and dome direction of aneurysm, the configuration of the cisternal space, the presence of preexisting hematomas, amount of the bleeding, and the cerebrospinal fluid flow2,8). Those collections of extraluminal contrast adjacent to ruptured aneurysm are needs to be differentiated from irregular blebs, calcification of the aneurysm and the artery, veins of variable thickness, and intra-aneurysmal thrombus14,25).
An ACoA aneurysm is often accompanied by IVH in the very act of bleeding. In theory, retrograde entry of SAH into the ventricular system is possible, but an unlikely cause of significant IVH. Recently, a report cited the lamina terminalis as the probable access for blood to the third ventricle; however the radiographic passage is not evidently from their CT images shown7). Investigators stated that the lamina terminalis does not make a strong resistance to hemorrhage from ACoA aneurysms, suggesting the lamina terminalis as an entry point from the interhemispheric fissure into the ventricular system4,16). However, the lamina terminalis is a solid membrane that requires sharp incision for anterior third ventriculostomy during surgery for tumors and aneurysms in this region. Furthermore, as confirmed in the present case, surgeons mentioned that direct penetration of the lamina terminalis has not been seen at clipping surgery for the ruptured ACoA aneurysms1,24). In this report, 3D CT images clearly illustrated the anatomical pathway of hemorrhage from ACoA aneurysm via the interhemispheric subarachnoid space through the lamina rostralis into the septum pellucidum and the frontal horn of the lateral ventricle.
Anatomically, the rostrum of the corpus callosum extends from the genu to the lamina terminalis, and consists of two sections : the thick beaked segment and the thin lamina rostralis. The lamina rostralis, the most rostral part of the corpus callosum, is visualized distinctly on spin-echo magnetic resonance images with 2 mm thickness9). The developing rostral corpus callosum from the rostral commissural plate seals the cavum septum pellucidum off from the anterior interhemispheric fissure during early fetal life20). Hence, the normally formed cavum septum pellucidum remains closed toward the anterior interhemispheric fissure. Because a cavum pellucidium regresses with maturation of the brain, the septum contains no open space in most human beings26). The lamina rostralis segment is superiorly continuous with the septum pellucidum and blends posteroinferiorly with the lamina terminalis just above the anterior commissure. The junction of the lamina rostralis and lamina terminalis is embedded within the very thin layers of the embryonic lamina reuniens segment of the lamina terminalis (Fig. 4).7,23) Consequently, in most individuals, the lamina rostralis secludes the anterior interhemispheric fissure from the imaginary interlaminar space within the septum pellucidum as well as the floor of the frontal horns. This filmy strip of nonmyelinated part of the rostrum is likely to offer less resistance to pressure than the lamina terminalis8,24). For that reason, passing through the lamina rostralis can give direct access from the subarachnoid spaces of the anterior interhemispheric fissure to the frontal horn of the lateral ventricle. In this case, the contrast extravasated during CTA was seen to penetrate into the frontal horn via the lamina rostralis through the caval-septal hematoma developed from the initial bleeding. The septal leaves may act as a buffer, containing the clot until the pressure within the closed intraseptal space is over their resistance, with subsequent extension into the lateral ventricle.
The previously reported patients with rebleeding around the time of CTA often had disturbances in consciousness and their prognosis was generally poor15,25). In contrast, this case showed a favorable recovery. For the selected patients with neurological responsiveness even after detection of extravasation on CTA, we should consider immediate and effective treatment.
References
1. Dehdashti AR, Rufenacht DA, Delavelle J, Reverdin A, de Tribolet N. Therapeutic decision and management of aneurysmal subarachnoid haemorrhage based on computed tomographic angiography. Br J Neurosurg. 2003; 17:46–53. PMID: 12779201.

2. Desai S, Friedman JA, Hlavin J, Kash F. Actively bleeding intracranial aneurysm demonstrated by CT angiography. Clin Neurol Neurosurg. 2009; 111:94–96. PMID: 18980796.

3. Fujii Y, Takeuchi S, Sasaki O, Minakawa T, Koike T, Tanaka R. Ultra-early rebleeding in spontaneous subarachnoid hemorrhage. J Neurosurg. 1996; 84:35–42. PMID: 8613833.

4. Gosselin MV, Vieco PT. Active hemorrhage of intracranial aneurysms : diagnosis by CT angiography. J Comput Assist Tomogr. 1997; 21:22–24. PMID: 9022763.

5. Hashiguchi A, Mimata C, Ichimura H, Morioka M, Kuratsu J. Rebleeding of ruptured cerebral aneurysms during three-dimensional computed tomographic angiography : report of two cases and literature review. Neurosurg Rev. 2007; 30:151–154. PMID: 17345112.

6. Holodny AI, Farkas J, Schlenk R, Maniker A. Demonstration of an actively bleeding aneurysm by CT angiography. AJNR Am J Neuroradiol. 2003; 24:962–964. PMID: 12748102.
7. Im SH, Oh CW, Hong SK, Kwon OK, Kim SH. CT angiography demonstration of the development of intraventricular hemorrhage during aneurysm rupture. Clin Neurol Neurosurg. 2007; 109:299–301. PMID: 17207925.

8. Kathuria S, Deveikis JP, Westesson PL, Gandhi D. Improved diagnosis of actively bleeding aneurysm on CT angiography using delayed CT images. Eur J Radiol. 2011; 79:328–331. PMID: 20227214.

9. Kier EL, Truwit CL. The lamina rostralis : modification of concepts concerning the anatomy, embryology, and MR appearance of the rostrum of the corpus callosum. AJNR Am J Neuroradiol. 1997; 18:715–722. PMID: 9127036.
10. Klisch J, Weyerbrock A, Spetzger U, Schumacher M. Active bleeding from ruptured cerebral aneurysms during diagnostic angiography: emergency treatment. AJNR Am J Neuroradiol. 2003; 24:2062–2065. PMID: 14625234.
11. Kuhn J, Vehlen C, Mennel HD, Mahkorn D, Bewermeyer H. Rupture of an internal carotid artery aneurysm during angiography with leakage of contrast medium via an external ventricular drain. Neuroradiology. 2003; 45:905–907. PMID: 14534767.

12. Kusumi M, Yamada M, Kitahara T, Endo M, Kan S, Iida H, et al. Rerupture of cerebral aneurysms during angiography--a retrospective study of 13 patients with subarachnoid hemorrhage. Acta Neurochir (Wien). 2005; 147:831–837. PMID: 15900400.

13. Larsen CC, Astrup J. Rebleeding after aneurysmal subarachnoid hemorrhage : a literature review. World Neurosurg. 2013; 79:307–312. PMID: 22722033.

14. Nagai M, Koizumi Y, Tsukue J, Watanabe E. A case of extravasation from a cerebral aneurysm during 3-dimensional computed tomography angiography. Surg Neurol. 2008; 69:411–413. PMID: 18261775.

15. Naidech AM, Janjua N, Kreiter KT, Ostapkovich ND, Fitzsimmons BF, Parra A, et al. Predictors and impact of aneurysm rebleeding after subarachnoid hemorrhage. Arch Neurol. 2005; 62:410–416. PMID: 15767506.

16. Nakatsuka M, Mizuno S, Uchida A. Extravasation on three-dimensional CT angiography in patients with acute subarachnoid hemorrhage and ruptured aneurysm. Neuroradiology. 2002; 44:25–30. PMID: 11942496.

17. Ohkuma H, Tsurutani H, Suzuki S. Incidence and significance of early aneurysmal rebleeding before neurosurgical or neurological management. Stroke. 2001; 32:1176–1180. PMID: 11340229.

18. Pérez-Núñez A, Alén JF, Ramos A, Millán JM. Aneurysm re-rupture during computed tomography angiography. Acta Radiol. 2006; 47:419–421. PMID: 16739704.

19. Saitoh H, Hayakawa K, Nishimura K, Okuno Y, Murayama C, Miyazawa T, et al. Intracarotid blood pressure changes during contrast medium injection. AJNR Am J Neuroradiol. 1996; 17:51–54. PMID: 8770249.
20. Scholtes F, Signorelli F, Bojanowski MW. Rupture of anterior communicating artery aneurysms during computed tomography angiography : description of the pathway for intraseptal and intraventricular hemorrhage. J Neurosurg. 2011; 115:617–620. PMID: 21599449.

21. Sorimachi T, Takeuchi S, Koike T, Minakawa T, Tanaka R. Intra-aneurysmal pressure changes during angiography in coil embolization. Surg Neurol. 1997; 48:451–457. PMID: 9352808.

22. Starke RM, Kim GH, Fernandez A, Komotar RJ, Hickman ZL, Otten ML, et al. Impact of a protocol for acute antifibrinolytic therapy on aneurysm rebleeding after subarachnoid hemorrhage. Stroke. 2008; 39:2617–2621. PMID: 18658042.

23. Tsuang FY, Su IC, Chen JY, Lee JE, Lai DM, Tu YK, et al. Hyperacute cerebral aneurysm rerupture during CT angiography. J Neurosurg. 2012; 116:1244–1250. PMID: 22443505.

24. Westerlaan HE, van Dijk JM, Jansen-van der Weide MC, de Groot JC, Groen RJ, Mooij JJ, et al. Intracranial aneurysms in patients with subarachnoid hemorrhage : CT angiography as a primary examination tool for diagnosis--systematic review and meta-analysis. Radiology. 2011; 258:134–145. PMID: 20935079.

25. Wu TC, Tsui YK, Chen TY, Lin CJ, Wu TC, Tzeng WS. Rebleeding of aneurysmal subarachnoid hemorrhage in computed tomography angiography : risk factor, rebleeding pattern, and outcome analysis. J Comput Assist Tomogr. 2012; 36:103–108. PMID: 22261779.

26. Yanaka K, Meguro K, Narushima K, Takano S, Doi M, Nose T. Basal perforating artery aneurysm within the cavum septi pellucidi. Case report. J Neurosurg. 1998; 88:601–604. PMID: 9488321.
Fig. 1
A : Initial plain CT scan shows thin SAH and subdural hematoma. B : There is no cavum septum pellucidum on the vertex cut. C : Source image of CTA reveals saccular aneurysms at the anterior communicating artery (arrow) and the left middle cerebral artery. D and E : Delayed images immediately after CTA show extensive SAH associated with intracerebral and intraventricular hemorrhages as well as blood within the septum pellucidum. CTA : computed tomography angiography, SAH : subarachnoid hemorrhage.

Fig. 2
A : Three-dimensional reconstruction of CTA depicts the spatial relationship between the aneurysm (arrow) and extravasated contrast medium (arrowhead). B : CTA with maximum intensity projection outlines the entire region of extravasated fresh blood (arrowheads) spouting from the ruptured ACoA aneurysm (arrow). ACoA : anterior communicating artery, CTA : computed tomography angiography.
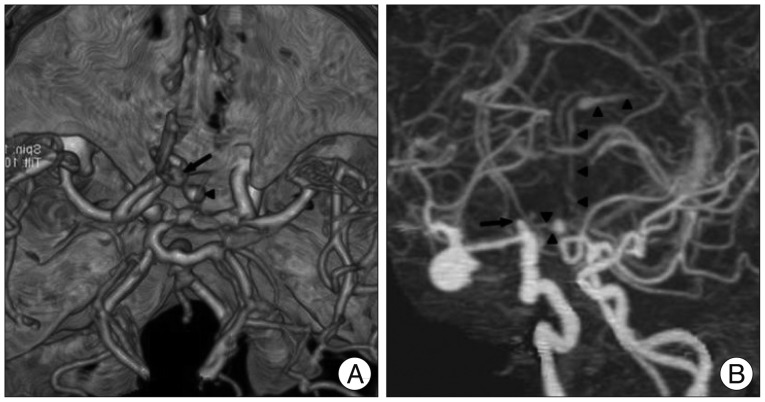
Fig. 3
A-D : Axial images of CTA demonstrate the caudorostral pathway of the extravasated contrast medium (arrowheads) into the preexisting hematoma of lower density from the interhemispheric cistern into the septum pellucidum and into the frontal horn and body of the lateral ventricle. E and F : Sagittal CTA views illustrate the diffusion of the contrast medium (arrowheads) from the rebleeding ACoA aneurysm (arrow) through the lamina rostralis (asterisk) into the septum pellucidum without penetrating into the lamina terminalis. G and H : Coronal reformatted CT scan clearly shows the ejected blood (arrowheads) which pass through the septum pellucidum up to the frontal horn of the left lateral ventricle. ACoA : anterior communicating artery, CTA : computed tomography angiography.
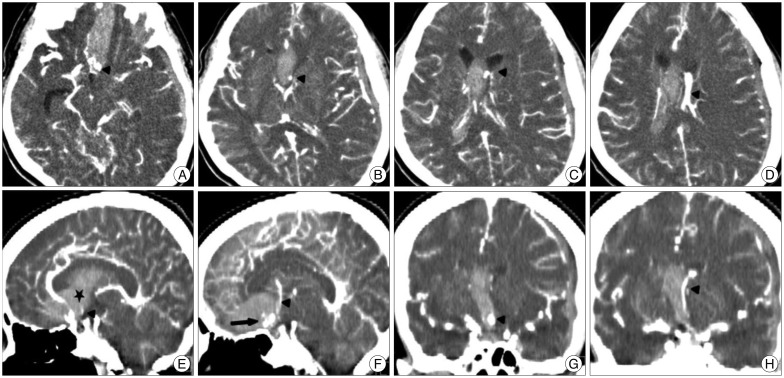
Fig. 4
Coronal T2-weighted magnetic resonance image shows the thin strip of white matter of the lamina rostralis (arrowhead). This separates the anterior interhemispheric cistern (lower arrow) from the space between the leaves of the septum pellucidum (upper arrow) as well as the floor of the frontal horns in the mature brain.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download