Abstract
Objective
Differentiation of demyelination in white matter from axonal damage can be determined using diffusion tensor imaging (DTI). In this study using meningioma patients an attempt was made to evaluate the relationship between preoperative weakness and the changes of diffusion parameters in the corticospinal tract (CST) using DTI.
Methods
Twenty-six patients with meningioma were enrolled in this study. Eleven of them suffered from objective motor weakness and were classified as Group 1. The remaining 15 patients did not present motor weakness and were classified as Group 2. Fiber tractography and CST diffusion parameters were obtained using DTIStudio. The ratios (lesion side mean value/contralateral side mean value) of CST diffusion parameters were compared with 1.0 as a test value using a one-sample t-test.
Results
In Group 1, fractional anisotropy (FA), tensor trace (TT), and radial diffusivity (RD, λ2 and λ3) of the CST were significantly different between two hemispheres, but axial diffusivity (AD, λ1) of the CST was not significantly different between two hemispheres. In Group 2, FA and λ3 of CST did not differ significantly between the hemispheres. In Group 2, TT, λ1, and λ2 of CST in the ipsilateral hemisphere were significantly higher than those of the unaffected hemisphere. However, the differences were small.
Diffusion tensor imaging (DTI) is a magnetic resonance imaging (MRI) technique that is sensitive to the diffusion properties of water molecules. The diffusion parameters describing the brain's microstructure include three eigenvalues (λ1, λ2, and λ3), the directionally averaged diffusion coefficient [apparent diffusion coefficient (ADC)], and fractional anisotropy (FA). The primary eigenvalue [λ1, axial diffusivity (AD)] is the magnitude of diffusivity in the direction of the primary eigenvector. In regions dominated by highly ordered axonal bundles, the primary eigenvalue describes the diffusivity parallel to the axonal bundles, whereas the second and third eigenvalues [λ2 and λ3, radial diffusivity (RD)] describe the diffusivity perpendicular to the axonal bundles. ADC is the mean of the three eigenvalues:
ADC=1/3 (λ1+λ2+λ3).
The tensor trace (TT) is the sum of the three eigenvalues :
TT=λ1+λ2+λ3.
ADC and TT describe the spatial diffusivity of water in a voxel. FA measures the degree of directionality of diffusion within a voxel2).
Demyelination and axonal damage are the hallmarks of white matter injury. A distinction between the two pathological findings is important. No noninvasive biological marker that can differentiate the white matter pathological findings of demyelination from axonal damage was available until the development of DTI9). Song et al.17) demonstrated that the water diffusivity perpendicular to an axonal fiber (RD) was significantly higher in shiverer mice than in their age-matched controls, reflecting a lack of myelin and increased freedom of cross-fiber diffusion in the white matter. Shiverer mice are homozygous for an autosomal recessive mutation in myelin basic protein, and are characterized by incomplete myelin formation in the central nervous system. In shiverer mice, the water diffusivity parallel to the axonal fiber tracts (AD) is not altered, which is consistent with the presence of intact axons. Budde et al.3) also suggested that a reduction in AD demonstrated with DTI is a specific marker of axonal damage in the spinal cord white matter of mice with experimental autoimmune encephalitis.
The altered states of the white matter that result from cerebral neoplasms might be expected to influence the measurement of DTI anisotropy and orientation in various ways. However, most studies with DTI in brain tumor patients have used only a qualitative approach to assess changes in the reconstructed fiber bundles.
Kim et al.6) demonstrated that the presence of motor weakness is significantly related to a low mean FA in the corticospinal tract (CST) on the lesion side. Based on their data, Kim et al.6) suggested that weakness might be quantitatively assessable by DTI. To our knowledge, study of the directional diffusivity in the white matter tract of meningioma patients has not been conducted.
We undertook a quantitative assessment with fiber tracking focusing on directional diffusivity of CST in meningioma patients. The purpose of this study was to evaluate the relationship between preoperative motor weakness and change of the diffusion in the CST of meningioma patients.
Twenty-six patients (6 men and 20 women) with meningioma were enrolled in this study. These patients had not undergone cranial surgery, brain radiation, or chemotherapy before DTI and MRI. We excluded patients with multiple meningiomas or meningiomas located in both hemispheres. Patients with falcine or parasagittal meningiomas located in one hemisphere were included.
Eleven patients suffered from objective motor weakness confirmed by neurological examination and were classified as Group 1. The remaining 15 patients did not report motor weakness, but had other symptoms, such as headache, memory disturbance, personality change, or seizure. These patients were classified as Group 2. The pathological diagnoses were seven benign meningiomas, two atypical meningiomas, and two anaplastic meningiomas in Group 1, and 12 benign meningiomas and three atypical meningiomas in Group 2.
The meningiomas in Group 1 comprised three right-hemisphere lesions and eight left-thoses. Two of the right-sided lesions were located in the convexity and one was falcine. Of the left-sided lesions, one was located in the convexity, three were falcine, three were parasagittal, and one was located on the sphenoid ridge. The meningioma in Group 2 comprised nine right-hemisphere lesions and six left-thoses. Of the right-sided lesions, four were located in the convexity, one was falcine, two were petroclival, and two were on the sphenoid ridge. Of the left-sided lesions, two were located on the convexity, one on the sphenoid ridge, one on the tuberculum sellae, one parasagittal, and one was tentorial.
DTIs were acquired using a 1.5 tesla (T) MRI system (Signa Excite, GE, Pewaukee, WI, USA) in two patients, a 3 T MRI system (Signa Excite, GE, Pewaukee, WI, USA) in four, and a 3 T MRI system (Magnetom Verio, Siemens, Erlangen, Germany) in five, using a conventional head gradient coil. We used a single-shot spin echo-echo planar imaging sequence. The b-factor was set at 1000 s/mm2 in seven patients and 700 s/mm2 in four. The acquisition parameters used were as follows. The field of view was 240×240 mm in seven patients and 220×220 mm in four. The matrix and gap were 256×256 and 0 mm in all patients. The repetition time (TR) was 10000-12400 ms and the echo time (TE) was 77-86 ms. To describe the intensity and direction of the diffusion anisotropy, the MR images were acquired with 13 noncollinear diffusion gradients (total slice number 35 or 36, slice thickness 3.5 mm) and without in two patients, with 25 noncollinear diffusion gradients (total slice number 38 or 39, slice thickness 3.0 or 3.5 mm) and without in four patients, and with 30 noncollinear diffusion gradients (total slice number 60, slice thickness 1.9 mm) and without in five patients.
DTIs were acquired using a 1.5 T MRI system (Signa Excite, GE) in one patient, a 3 T MRI system (Signa Excite, GE) in five, a 1.5 T MR (Signa Excite HDx, GE) in one patient, a 3 T MRI system (Magnetom Trio, A Tim, Siemens) in one, and a 3 T MRI system (Magnetom Verio, Siemens) in seven, using a conventional head gradient coil. We used a single-shot spin echo-echo planar imaging sequence. The b-factor was set at 1000 s/mm2 in 12 patients and 700 s/mm2 in three. The acquisition parameters used were as follows. The field of view was 240×240 mm in 12, 230×230 mm in one patient, and 220×220 mm in two. The matrix was 256×256 in 14 patients and 128×128 in one. The gap was 0 mm in all patients. TR was 10000-11400 ms and TE was 74-86 ms. To describe the intensity and direction of the diffusion anisotropy, MR images were acquired with 13 noncollinear diffusion gradients (total slice number 35 or 36, slice thickness 3.5 mm) and without in two patients, with 25 noncollinear diffusion gradients (total slice number 38 or 39, slice thickness 3.0 or 3.5 mm) and without in five patients, and with 30 noncollinear diffusion gradients (total slice number 60, slice thickness 1.9 mm) and without in eight patients.
In Groups 1 and 2, from 13, 25, and 30 diffusion-weighted images, we obtained six diffusion tensor components by using multiple-order linear equations: DXX, DYY, DZZ, DXY, DXZ, and DYZ. To encode the fiber tract directions, we used the maximum eigenvalue (Vmax) of the three eigenvectors obtained by the DTI eigen decomposition.
Fiber tractography was performed using fiber assignment with the continuous tracking method proposed by Mori et al.8). A continuous tracking algorithm using DTIStudio (Processing Tools and Environment for DTI, ver. 2, H. Jiang and S. Mori) was used in which the path follows the principal eigenvector of the diffusion tensor on a subvoxel level until the voxel edge is met, at which point the direction abruptly changes to that of the new voxel.
FA mapping was transformed into a color code : anterior to posterior, green; superior to inferior, blue; and left to right, red. The tracking algorithm was initiated from a user-defined "seed" region of interest (ROI). Tracking was initiated in both retrograde and orthograde directions according to the direction of the principal eigenvector in the ROI.
The seed ROI for the CST was the posterior limb of the internal capsule (PLIC). The target ROI was the ipsilateral cerebral peduncle. The seed and target ROIs were selected manually on directionally encoded colored maps. Only tracts starting from the seed ROI and passing through the target ROI were included in the trace of the CST (Fig. 1).
Tracking of the CST was terminated when the fibers showed a steep turn (more than 70°). Tracking the CST was also terminated when the threshold of the FA level was below 0.2.
Following anatomy-based tractography, a further quantitative analysis of the identified displaced fiber tract was performed. FA, TT, and the three eigenvalues of the CST were studied using the "tract statistics" function of DTIStudio, which allows a statistical evaluation of the diffusion parameters of the reconstructed fibers.
Tumor size and patient's age between Groups 1 and 2 were compared using a Mann-Whitney U test. The ratios (lesioned side mean value/contralateral side mean value) of all the diffusion parameters for the CST on DTI were compared with 1.0 as the test value using a one-sample t-test. The statistical analysis was performed with the SPSS software package (version 14.0; SPSS Inc., Chicago, IL, USA). Statistical significance was accepted for probability values of less than 0.05.
All of diffusion parameters are presented as graphs (Fig. 2, 3). All data are presented as mean±standard deviation (SD). In each graph, the values represented by the vertical line and box are as follows : lowest level of the vertical line is the lowest value; highest level of the vertical line is the highest value; bottom of the box is the mean-one SD; top of the box is the mean+one SD.
In Group 1, FA of the CST in the hemisphere ipsilateral to the meningioma was significantly lower than that in the contralateral hemisphere (14% decrease). AD (λ1) of the CST in Group 1 did not differ significantly between the hemispheres. In Group 1, TT (15% increase) and RD (λ2 and λ3, 28-33% increase) for the CST in the hemisphere affected by the meningioma were significantly higher than those for the unaffected hemisphere (Fig. 2).
In Group 2, FA (3% decrease) and λ3 (10% increase) of CST did not differ significantly between the two hemispheres, whereas TT (4% increase), λ1 (3% increase), and λ2 (10% increase) in the CST of the ipsilateral hemisphere were significantly higher than those of the unaffected hemisphere. However, the degree of difference was small (range of 3-10% increase) (Fig. 3).
Several studies have shown that the fiber tracts in the vicinities of brain tumors can be displaced, disrupted, or invaded by the tumor1,4,14,15). Previous reports indicated that white matter FA in brain tumor patients might be increased, unchanged, or decreased5,6,14,18). The clinical significance of these changes in FA is unclear. Although the diffusion technique opens new ways to investigate cerebral damage in brain tumors, research investigating the relationship between DTI-derived measures and motor weakness in brain tumor patients is scarce.
Song et al.17) reported that DTI results described the effects of dysmyelination on the water directional diffusivities in the brains of shiverer mice. Transverse diffusivity refers to the diffusion across fibers rather than along the fibers and is believed to be a specific marker for demyelination19). By contrast, a decrease in axial diffusivity, the diffusion of water parallel to white matter fibers, is indicative of axonal damage7,16).
Recently, a strong correlation between diffusion-derived measures and motor weakness was found in patients with brain tumors6). Helton et al.5) found decreased FA and increased ADC of CST and transverse pontine fibers in children with diffuse pontine gliomas. These authors described that a comparison of the severity of cranial nerve deficits with the average FA of the transverse pontine tract showed a marginal difference (p=0.057). Nilsson et al.10) reported that there were no significant differences in FA, ADC, and eigenvalues between white matter adjacent to low-grade tumors and on the contralateral side. Furthermore, they suggested that the microstructural integrity of the white matter adjacent to the tumor was preserved. However, they studied only low-grade tumor patients with no neurological deficit. Although the study by Nilsson et al.10) did not evaluate meningioma patients, their finding is consistent with our data from patients without weakness.
To our knowledge, no previous study of the correlation between directional diffusivity and motor weakness in meningioma patients has been reported. We have demonstrated that preoperative weakness in meningioma patients can be indicated by DTI data as reduced FA and elevated TT values. On the basis of our eigenvalue data, it can also be argued that the elevated TT values we observed in meningioma patients with weakness are attributable to increased diffusion perpendicular to the CST (as indicated by the increased λ2 and λ3 values). These results are similar to those observed with demyelination. Perhaps there are subtle demyelination in the "so called purely vasogenic edema" associated with meningioma that have previously been unrecognized and that now can be detected by DTI.
Axonal fiber packing or compression by the tumor will affect both AD and RD. However, it is difficult to predict how this packing will affect the directional diffusivities17). A study by Schonberg et al.14) showed that along fiber systems that are displaced and compressed as a result of brain tumors, the diffusivity increases parallel to the fibers and decreases perpendicular to them. This leads to an overall increase in FA and to the conclusion that this may reflect the compression of the fiber bundle14). The results of Schonberg et al.14) are contrary to our data; however, their study did not consider weakness when evaluating FA and TT measurements in the peritumoral white matter.
The dependence of FA on age has been described in a study by Salat et al.13), who reported a reduction in FA of 0.2% per year within the PLIC. Yasmin et al.20) evaluated FA of the bilateral CSTs of 100 healthy subjects and demonstrated no asymmetry. Yasmin et al.20) also reported that the left-right ratio of mean diffusivity in the bilateral CST ranged from 0.9726 to 0.9736 (p<0.0001). Therefore, we can ignore left-right asymmetry in the diffusion parameters of the CST in healthy subjects.
Our methods have some limitations. First, image distortion and the relatively lower signal-to-noise ratio of DTI represent major problems. The second limitation is that image reconstruction by tractography is not a precise stepwise procedure with a reproducible outcome, but is dependent on the manipulation of the ROIs. Because of these limitations, we believe that the quantitative estimation of the CST with fiber tracking may not be accurate. Tracking results may change in regions with lower FA, such as tumor-infiltrated areas or areas of crossing fibers, where the CST intersects with callosal fibers at the level of the centrum semiovale11). In our study, fiber tracking was unable to resolve crossing fiber pathways.
The relationship between demyelination and increase in RD is somewhat more complex for a variety of reasons. For instance, it is not yet clear whether RD is specific to demyelination in the presence of significant axonal damage or inflammation3).
In this study, we used several types of MRI systems and several kinds of MR imaging acquisition parameters for DTI. We used two methods to verify that the different MRI systems and parameters did not significantly influence the diffusion parameters. We evaluated the ratios of diffusion parameters in both hemispheres of same patient. We also examined whether the diffusion parameters of CST in contralateral (healthy) side in our study had values similar to those reported in other studies. Reich et al.12) demonstrated that the mean of FA, TT, λ1, λ2, and λ3 were 0.58, 0.00237, 0.0014, 0.00062, and 0.00038 in healthy individuals CST. In our results, mean of FA, TT, λ1, λ2, and λ3 were 0.5976, 0.00238, 0.0014, 0.0006, and 0.000358 in contralateral (healthy) side CST. Our diffusion parameter results for CST were compatible with the results of Reich et al.12) despite the several types of MRI systems and several kinds of acquisition parameters for MR imaging used.
We have demonstrated that preoperative weakness in meningioma patients can be indicated by DTI data as reduced FA and elevated TT values. On the basis of our eigenvalue data, it can also be argued that the elevated TT values we observed in meningioma patients with weakness are attributable to increased diffusion perpendicular to the CST. These results suggest that the diffusion parameters of CST in hemisphere affected by meningioma in patient with weakness were partly influenced by demyelination.
Acknowledgements
This work was supported by research grant from a Seoul National University College of Medicine.
References
1. Bello L, Gambini A, Castellano A, Carrabba G, Acerbi F, Fava E, et al. Motor and language DTI Fiber Tracking combined with intraoperative subcortical mapping for surgical removal of gliomas. Neuroimage. 2008; 39:369–382. PMID: 17911032.

2. Berman JI, Mukherjee P, Partridge SC, Miller SP, Ferriero DM, Barkovich AJ, et al. Quantitative diffusion tensor MRI fiber tractography of sensorimotor white matter development in premature infants. Neuroimage. 2005; 27:862–871. PMID: 15978841.

3. Budde MD, Xie M, Cross AH, Song SK. Axial diffusivity is the primary correlate of axonal injury in the experimental autoimmune encephalomyelitis spinal cord : a quantitative pixelwise analysis. J Neurosci. 2009; 29:2805–2813. PMID: 19261876.

4. Field AS, Alexander AL, Wu YC, Hasan KM, Witwer B, Badie B. Diffusion tensor eigenvector directional color imaging patterns in the evaluation of cerebral white matter tracts altered by tumor. J Magn Reson Imaging. 2004; 20:555–562. PMID: 15390227.

5. Helton KJ, Phillips NS, Khan RB, Boop FA, Sanford RA, Zou P, et al. Diffusion tensor imaging of tract involvement in children with pontine tumors. AJNR Am J Neuroradiol. 2006; 27:786–793. PMID: 16611765.
6. Kim CH, Chung CK, Kim JS, Jahng TA, Lee JH, Song IC. Use of diffusion tensor imaging to evaluate weakness. J Neurosurg. 2007; 106:111–118. PMID: 17236496.

7. Loy DN, Kim JH, Xie M, Schmidt RE, Trinkaus K, Song SK. Diffusion tensor imaging predicts hyperacute spinal cord injury severity. J Neurotrauma. 2007; 24:979–990. PMID: 17600514.

8. Mori S, Frederiksen K, van Zijl PC, Stieltjes B, Kraut MA, Solaiyappan M, et al. Brain white matter anatomy of tumor patients evaluated with diffusion tensor imaging. Ann Neurol. 2002; 51:377–380. PMID: 11891834.

9. Nagesh V, Tsien CI, Chenevert TL, Ross BD, Lawrence TS, Junick L, et al. Radiation-induced changes in normal-appearing white matter in patients with cerebral tumors : a diffusion tensor imaging study. Int J Radiat Oncol Biol Phys. 2008; 70:1002–1010. PMID: 18313524.

10. Nilsson D, Rutka JT, Snead OC 3rd, Raybaud CR, Widjaja E. Preserved structural integrity of white matter adjacent to low-grade tumors. Childs Nerv Syst. 2008; 24:313–320. PMID: 17960393.

11. Okada T, Mikuni N, Miki Y, Kikuta K, Urayama S, Hanakawa T, et al. Corticospinal tract localization : integration of diffusion-tensor tractography at 3-T MR imaging with intraoperative white matter stimulation mapping-preliminary results. Radiology. 2006; 240:849–857. PMID: 16857980.

12. Reich DS, Smith SA, Jones CK, Zackowski KM, van Zijl PC, Calabresi PA, et al. Quantitative characterization of the corticospinal tract at 3T. AJNR Am J Neuroradiol. 2006; 27:2168–2178. PMID: 17110689.
13. Salat DH, Tuch DS, Greve DN, van der Kouwe AJ, Hevelone ND, Zaleta AK, et al. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol Aging. 2005; 26:1215–1227. PMID: 15917106.

14. Schonberg T, Pianka P, Hendler T, Pasternak O, Assaf Y. Characterization of displaced white matter by brain tumors using combined DTI and fMRI. Neuroimage. 2006; 30:1100–1111. PMID: 16427322.

15. Smits M, Vernooij MW, Wielopolski PA, Vincent AJ, Houston GC, van der Lugt A. Incorporating functional MR imaging into diffusion tensortractography in the preoperative assessment of the corticospinal tract in patients with brain tumors. AJNR Am J Neuroradiol. 2007; 28:1354–1361. PMID: 17698540.

16. Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003; 20:1714–1722. PMID: 14642481.

17. Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002; 17:1429–1436. PMID: 12414282.

18. Stadlbauer A, Ganslandt O, Buslei R, Hammen T, Gruber S, Moser E, et al. Gliomas : histopathologic evaluation of changes in directionality and magnitude of water diffusion at diffusion-tensor MR imaging. Radiology. 2006; 240:803–810. PMID: 16926329.

19. Warlop NP, Achten E, Fieremans E, Debruyne J, Vingerhoets G. Transverse diffusivity of cerebral parenchyma predicts visual tracking performance in relapsing-remitting multiple sclerosis. Brain Cogn. 2009; 71:410–415. PMID: 19576672.

20. Yasmin H, Aoki S, Abe O, Nakata Y, Hayashi N, Masutani Y, et al. Tract-specific analysis of white matter pathways in healthy subjects : a pilot study using diffusion tensor MRI. Neuroradiology. 2009; 51:831–840. PMID: 19662389.

Fig. 1
Corticospinal tract (CST) fiber tracking in a 37-year-old man with benign meningioma. A 37-year-old man presented with seizure and right-upper-extremity weakness. A T1-enhanced image shows a slightly enhanced mass in the left frontal lobe (A). The posterior limb of the internal capsule is selected as the seed region of interest (ROI) (B and C). The ipsilateral cerebral peduncle is selected as the target ROI (D). The tractography image shows the CST on a coronal T2-weighted image (E).
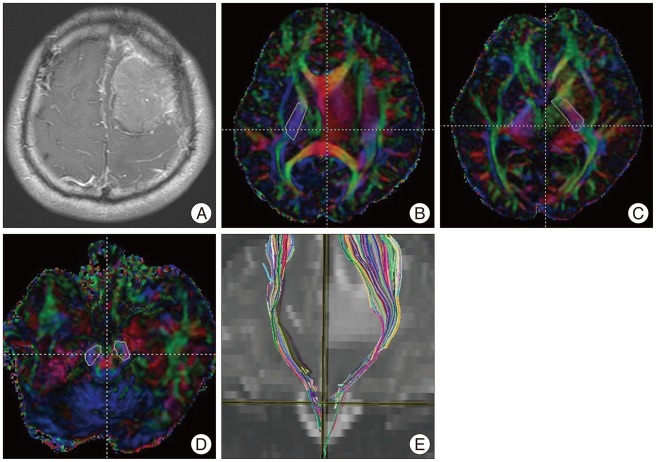
Fig. 2
Ratios (lesion/contralateral) of the diffusion parameters (FA, TT, λ1, λ2, and λ3) for the CST in meningioma patients with weakness (Group 1). y-axis : ratio of diffusion parameters for the CST in the two hemispheres, FA : fractional anisotropy, TT : tensor trace, 1 : primary eigenvalue, λ2 : second eigenvalue, λ3 : third eigenvalue.
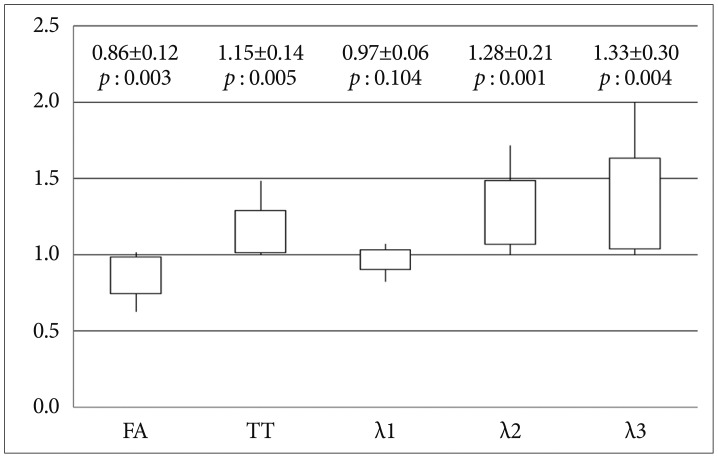
Fig. 3
Ratios (lesion/contralateral) of diffusion parameters (FA, TT, λ1, λ2, and λ3) for the CST in meningioma patients without weakness (Group 2). y-axis : ratio of diffusion parameters for the CST in the two hemispheres, FA : fractional anisotropy, TT : tensor trace, λ1 : primary eigenvalue, λ2 : second eigenvalue, λ3 : third eigenvalue.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download