Abstract
Objective
Symptomatic disc degeneration develops from inflammatory reactions in the annulus fibrosus (AF). Although inflammatory mediators during annular inflammation have been studied, the roles of matrix metalloproteinases (MMPs) and their inhibitors have not been fully elucidated. In this study, we evaluated the production of MMPs and tissue inhibitors of metalloproteinase (TIMPs) during annular inflammation using an in vitro co-culture system. We also examined the effect of notochordal cells on annular inflammation.
Methods
Human AF (hAF) pellet was co-cultured for 48 hours with phorbol myristate acetate-stimulated macrophage-like THP-1 cells. hAF pellet and conditioned media (CM) from co-cultured cells were assayed for MMPs, TIMPs, and insulin-like growth factor (IGF)-1 levels using real-time reverse-transcriptase polymerase chain reaction and enzyem-linked immunosorbent assay. To evaluate whether notochordal cells affected MMPs or TIMPs production on annular inflammation, hAF co-cultured with notochordal cells from adult New Zealand White rabbits, were assayed.
Results
MMP-1, -3, -9; and TIMP-1 levels were significantly increased in CM of hAF co-cultured with macrophage-like cells compared with hAF alone, whereas TIMP-2 and IGF-1 levels were significantly decreased (p<0.05). After macrophage exposure, hAF produced significantly more MMP-1 and -3 and less TIMP-1 and -2. Interleukin-1β stimulation enhanced MMP-1 and -3 levels, and significantly diminished TIMP-2 levels. Co-culturing with rabbit notochordal cells did not significantly influence MMPs and TIMPs production or COL1A2 gene expression.
Conclusion
Our results indicate that macrophage-like cells evoke annular degeneration through the regulation of major degradative enzymes and their inhibitors, produced by hAF, suggesting that the selective regulation of these enzymes provides future targets for symptomatic disc degeneration therapy.
Approximately 80% of the population experience low back pain during their lives2). Recent studies have demonstrated that symptomatic disc degeneration is the most important cause of low back pain15). Peng et al.16) found that symptomatic discs had distinct pathological characteristics, including the formation of vascularized tissue and abundant macrophages along a torn fissure of the annulus fibrosus (AF). We previously demonstrated that macrophages play a major role in the production of inflammatory mediators from the AF, and that these inflammatory mediators might be related to discogenic low back pain6). In addition to the production of inflammatory mediators, interactions between the AF and macrophages could lead to symptomatic degeneration of the AF via a vicious cycle in the regulation of the extracellular matrix (ECM).
The ECM of the AF is rich in collagens, especially type I collagen18). It is known that ECM remodeling is enabled by the regulation of matrix metalloproteinases (MMPs) and "tissue inhibitors of metalloproteinases" (TIMPs)7). It has been proposed that macrophages infiltrating within the discs is an early step before ECM degradation, initiated by MMPs22). Among the large number of MMPs (now, at least 24 types), MMP-1 (collagenase-1) is a unique protease that cleaves native interstitial collagen types I, II, and III, and MMP-3 (stromyelysin-1), primarily degrades non-collagenous matrix proteins, but also denatures collagens18,22). Recent biochemical analyses have shown that MMP-1 and -3 are increasingly expressed in degenerated disc tissue samples and have been considered to be a possible initial event in disc alterations3). MMP-9 cleaves denatured collagen molecules and is a key enzyme in inflammatory joint disease13). Despite these preliminary data, it remains unclear which MMP(s) are primarily responsible for matrix disruption in the AF during an acute inflammatory reaction.
Under normal physiological conditions, the efficiency of MMPs is regulated by their own activation mechanism as well as by endogenous TIMPs. TIMP-1 and -2 are the most important and widespread proteins involved in human MMP regulation17). Insulin-like growth factor (IGF)-1 cause proliferation and matrix synthesis in human nucleus pulposus (NP) cells, and are potential therapeutic tools for the treatment of disc degeneration9). Considered together, these factors might be involved in the regulation of ECM metabolism during annular inflammation.
Notochordal cells play an important role in the development of the vertebral column and discs during embryogenesis21). Previous studies have suggested that notochordal cells proliferate and synthesize ECM components, and stimulate matrix production from the NP via chondrocyte-like cells1,10). Co culturing of notochordal and AF cells revealed that notochordal cells activated AF cell metabolism and retarded disc degeneration when reinserted into the disc14). In a previous study, we showed that rabbit notochordal cells (rNC) cells reduced the expression of genes and the production of proinflammatory factors in macrophage-exposed AF cells12). However, whether rNC cells influence MMPs and TIMPs production during annular inflammation has not been studied. In this study, we used an in vitro co-culture system to investigate the pathomechanism of ECM regulation and to examine the effects of rNC cells on symptomatic disc degeneration.
Human AF (hAF) cells were isolated from the disc tissues (4 males, 3 females). The disc tissues were removed during elective surgery for degenerative spinal disease (grades II-III), according to regulations of our institutional review board. Tissue specimens were placed in sterile Ham's F-12 medium (Gibco-BRL, Grand Island, NY, USA) containing 1% penicillin/streptomycin (P/S; Gibco-BRL, Grand Island, NY, USA) and 5% fetal bovine serum (FBS; Gibco-BRL, Grand Island, NY, USA). After washing, the definitive hAF region was resected from the tissues and was digested for 60 min in F-12 medium containing 1% P/S, 5% FBS, and 0.2% pronase (Calbiochem, La Jolla, CA, USA), and then incubated overnight in 0.025% collagenase I (Roche Diagnostics, Mannheim, Germany). Filtered cells were centrifuged, resuspended, and cultured in F-12 medium that contained 10% FBS and 1% P/S (culture medium) in a humidified atmosphere of 5% CO2 at 37℃.
A 'pellet' culture system was used in this study to mimic the three-dimensional cellular interactions of the in vivo environment. 95%-confluent cells were removed from the flask by treatment with 0.05% trypsin/EDTA (Gibco-BRL, Grand Island, NY, USA), and the hAF cells (2×105/mL) were resuspended in culture medium. The cells were placed in individual 15-mL polypropylene conical tubes, centrifuged (5 min, 2000 rpm), and then incubated for 7 days.
Human acute monocytic leukemia (THP-1) cells (Korean Cell Line Bank, Seoul, Korea) belong to the mononuclear phagocyte series20) and were converted into activated macrophage-like cells by incubation with phorbol myristate acetate (PMA). Our previous results have shown that these activated macrophage-like cells produced pro-inflammatory factors6). In this experiment, THP-1 cells were maintained in RPMI 1640 medium (ATCC, Manassas, VA, USA), supplemented with 10% FBS, 1% P/S, and 0.05 mM 2-mercaptoethanol (Sigma-Aldrich, St. Louis, MO, USA) and stimulated to differentiate into macrophage-like THP-1 cells by treatment with 160 nM PMA (Sigma-Aldrich) for 72 hours before co-culturing.
To isolate rNC cells, discs were harvested from the spines of mature New Zealand white rabbits (4-6 months of age, -2.5 kg) immediately postmortem, in accordance with the guidelines of our Institutional Animal Care and Use Committee. NP tissues were dissected from the specimens, washed, digested for 40 min in F-12 medium containing 1% P/S, 5% FBS, and 0.2% pronase, and incubated for 4 h in 0.025% collagenase P (Sigma-Aldrich). Cells from the digested tissues were passed through a sterile nylon mesh, collected by centrifugation, and cultured in culture medium. As notochordal cell clusters do not adhere to the flasks until day 6 of culture5), the clusters were successfully separated from the chondrocyte-like cells on day 3.
PMA-activated macrophage-like THP-1 cells were removed from the culture flasks using trypsin treatment and then placed in a 24-well plate at a density of 1×105 cells/well in 1 mL F 12/Dulbecco's modified Eagle's medium with 1% FBS and 1% P/S (serum-starved medium). The hAF pellet was added to the cell culture insert (0.4-µm pore size; Becton Dickinson Labware, Franklin Lakes, NJ, USA) in each well. For co-culturing with notochordal cells, rNC cell clusters (2×104 clusters/well) were added to inserts that contained hAF pellets. Conditioned medium (CM) from 48-h co-cultured cells was collected for analysis in enzyme-linked immunosorbent assays (ELISAs). To assess enzyme levels produced by the hAF pellet co cultured with rNC cell clusters and macrophages, the co-cultured hAF pellet was moved to a new well and cultured in serum-starved medium. After 48 h of incubation, the hAF pellets and CM were removed and stored at -80℃.
To evaluate MMPs and TIMPs production in hAF pellet by IL-1β stimulation, naïve hAF pellets and macrophage-exposed hAF (nemotic hAF) pellets were cultured with 1 ng/mL recombinant human IL-1β (Sigma-Aldrich) for 48 h. After stimulation, the hAF pellets and CM were stored at -80℃.
Total RNA was extracted from the hAF pellet using the RNeasy Mini Kit (QIAGEN, Valencia, CA, USA). cDNA was synthesized from total RNA (1 µg) using a Maxim RT PreMix Kit (iNtRON, Seongnam, Korea). The reactions used the primers (Table 1) in triplicate in 96-well plates (25 µL) using the iTag SYBR Green Supermix in the ROX kit (Bio-Rad, Hercules, CA, USA) and an ABI Prism 7300 detection system (Applied Biosystems, Foster City, CA, USA). The threshold cycle (Ct) values were determined, and the data were normalized to those of the actin gene using the ΔΔ Ct method to calculate relative mRNA levels compared with untreated samples.
The level of MMP-1 was increased significantly in the CM of hAF pellets co-cultured with macrophage-like cells (hAF-M; 25.78±7.08 ng/mL), compared with the level produced by naïve hAF pellets (hAF; 0 ng/mL), whereas the level of MMP-1 did not differ significantly from that produced by macrophage-like cells (M; 28.26±10.29 ng/mL) alone (Fig. 2A). The levels of MMP-3 and TIMP-1 were increased significantly in the CM of hAF-M (MMP-3, 329.27±99.76 ng/mL; TIMP-1, 988.67±347.63 ng/mL), compared with the levels in the CM of hAF (MMP-3, 4.97±1.84 ng/mL; TIMP-1, 36.00±8.99 ng/mL) or M (MMP-3, 1.89±0.59 ng/mL; TIMP-1, 431.04±251.05 ng/mL) (Fig. 2B, C). The hAF (6.40±1.49 ng/mL) produced a higher level of TIMP-2 than did M (2.46±1.23 ng/mL) and hAF-M (4.69±1.21 ng/mL) (Fig. 2D). The level of MMP-9 was significantly higher in the CM of M (441.00±160.96 ng/mL) and hAF-M (285.23±27.84 ng/mL) cells than hAF (0.20±0.06 ng/mL) (Fig. 2E). The hAF pellet produced greater levels of IGF-1 (27.81±7.85 pg/mL). In contrast, IGF-1 levels from hAF-M (12.56±1.13 pg/mL) were significantly lower than those produced by hAF and M (17.99±3.047 ng/mL) (Fig. 2F).
After macrophage exposure, the nemotic hAF pellet [hAF(M)] produced significantly higher levels of MMP-1 (9.14±4.02 ng/mL) and -3 (118.26±42.25 ng/mL), and lower levels of TIMP-1 (14.83±6.74 ng/mL) and -2 (1.78±0.48 ng/mL), compared with naïve hAF (hAF; MMP-1, 0 ng/mL; MMP-3, 6.60±3.98 ng/mL; TIMP-1, 46.94±17.86 ng/mL; TIMP-2, 5.51±2.04 ng/mL) (Fig. 3). Stimulation with 1 ng/mL IL-1β enhanced the levels of MMP-1 and -3 in the hAF (MMP-1, 14.88±4.71 ng/mL; MMP-3, 412.71±151.60 ng/mL) and hAF(M) (MMP-1, 16.32±6.48 ng/mL; MMP-3, 235.47±92.86 ng/mL), although the hAF responded more sensitively than the hAF(M) to IL-1β stimulation (Fig. 3A, B). Stimulation with 1 ng/mL IL-1β tended to decrease the level of TIMP-1 and significantly diminish the level TIMP-2 in hAF (TIMP-1, 39.57±15.99 ng/mL; TIMP-2, 3.36±1.06 ng/mL) and hAF(M) pellets (TIMP-1, 14.83±6.74 ng/mL; TIMP-2, 1.78±0.48 ng/mL) (Fig. 3C, D).
To confirm the origin of MMP-3 and TIMP-1 in the co-culture group, levels of their mRNAs were measured in naïve hAF (hAF) and nemotic hAF [hAF(M)]. MMP-3 mRNA from hAF(M) was expressed at a level 2.5-fold higher than that of hAF, while TIMP-1 mRNA from hAF(M) was expressed at a level 9-fold lower than that of hAF (Fig. 4).
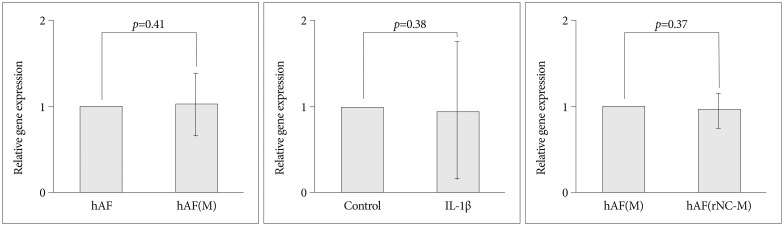
Compared with levels produced by hAF(M), co-culturing with rNC cells [hAF(rNC-M)] tended to decrease the levels of MMP-3 and TIMP-1, but did not influence the production of MMP-1 or TIMP-2 (Fig. 5), while rNC cells decreased the level of MMP-3 mRNA and increased the level of TIMP-1 mRNA in hAF(M) (Fig. 4). The hAF pellet expression of COL1A2 was not influenced by macrophage exposure, IL-1β stimulation, or rNC cell treatment in 48 h of culture (Fig. 6).
In various pathological processes, an imbalance between MMPs and TIMPs plays a major role in tissue degradation and decomposition. Biochemical changes that occur in the ECM of the intervertebral disc (IVD) have been investigated8), but their roles during annular inflammation, an early stage of symptomatic disc degeneration, are poorly understood. Our results suggest that macrophages play a major role in the development of symptomatic disc degeneration via the regulation of enzyme production by AF. Additionally, our results suggest that rabbit notochordal cells would not provide effective therapeutic regulation of these factors during annular inflammation.
MMP-1 and -3 are known to be stimulated at an early disease stage and to initiate matrix degradation3). MMP-1 breaks down the helical region of the fibrillar (types I and II) collagens, and MMP-3 has broad substrate specificity, degrading ECM proteoglycans and collagens following their initial degradation by collagenases19). Although many authors have reported the presence of MMPs from disc tissues, it was not confirmed that the AF itself, which is the primary pain perception organ, is capable of enhancing the production of MMP-1 and -3 after an inflammatory reaction. In this study, the naïve hAF pellet produce minimal level of MMP-1 and MMP-3. When hAF pellets were co-cultured with macrophages, the MMP-3 levels were significantly increased. Furthermore, co cultures treated with IL-1β for 48 h stimulated the hAF pellet to produce higher levels of MMP-3 than MMP-1. These results suggest that MMP-3, rather than MMP-1, plays a major role in the degradation of the AF after acute inflammation although MMP-1 is also an essential enzyme at the stage of AF infiltrated with macrophages.
In a clinical study of osteoarthritis (OA), the levels of MMP-9 in the plasma and serum correlated positively with the severity of damage to joint cartilage and subchondral bone11). Thus, MMP-9 could be a major enzyme involved in the annular inflammatory reaction. In this study, naïve hAF did not produce MMP-9, but macrophages did produce large amounts of MMP-9. Furthermore, macrophage and IL-1β stimulation did not induce hAF to produce MMP-9 (data not shown). These results suggest that MMP-9 could be a major MMP in AF during inflammation, but might not be an important MMP in the AF after inflammation.
We examined the levels of TIMP-1 and -2 produced by hAF when co-cultured with macrophages. Co-culturing produced high levels of TIMP-1, compared with TIMP-2, indicating that TIMP-1 is the major factor in the TIMP family during annular inflammation. Roberts et al.17) showed an increase in TIMP-1 immunoreactivity within degenerate discs while TIMP-2 was found at a similar level in all sample groups, indicating that TIMP-1 rather than TIMP-2 was the major inhibitory enzymes in disc degeneration. In previous molecular analyses of degenerative disc tissues, the pattern of TIMP-1 paralleled that of MMPs although TIMPs have inhibitory actions against MMPs3,8). However, they did not confirm the origin of TIMP-1, because they used the disc tissues probably with infiltrating macrophages. In the present study, TIMP-1 production and mRNA from AF was decreased by macrophage exposure or IL-1β stimulation, although the pattern of TIMP-1 production was similar to that of MMP-3 during co-culture. These results suggest that most of the TIMP 1 during degeneration come from macrophages, and AF cells produced TIMPs and MMPs in a contradictory pattern as counterpart enzymes.
In the present study, TIMP-2 and IGF-1 were significantly decreased in co-cultured cells compared with naïve hAF. IGF-1 is an important growth factor for the homeostasis of cells and has anabolic effects, by stimulating the synthesis of ECM proteins4). TIMP-2 may be also critical for the maintenance of tissue homeostasis by directly suppressing the proliferation of quiescent tissues in response to angiogenic factors, a unique role within the TIMP family19). Together with previous reports, our data suggest that interactions between AF and macrophages induce stressful conditions that disturb the maintenance of tissue homeostasis in the AF via suppression of TIMP-2 and IGF-1.
In the degenerative disc model, notochordal cells interact closely with chondrocyte-like cells through the supportive functions of anabolic tissue growth factors, secreted by notochordal cells. Although notochordal cells exert a positive effect on the development of symptomatic disc degeneration, it is practically impossible to obtain human notochordal cells, because they typically disappear in humans within the first decade of life and are replaced by non-notochordal (chondrocyte-like) cells. By contrast, the notochordal cells of rabbit IVD persist into adulthood and produce 4 to 5 times more glycosaminoglycan than do non-notochordal cells. Furthermore, as the IVD is an avascular tissue, the risk of immune complications is low, which means that tissue rejection should not be a significant issue in the transplantation of notochordal cells into human IVD. Previously, we confirmed that rNC cells have an anti-inflammatory function during annular inflammation12). Thus, we hypothesized that notochordal cells might have the ability to suppress MMPs and increase TIMPs for the prevention of collagen degradation during annular inflammation. In our study, notochordal cells decreased the MMP-3 mRNA and increased TIMP-1 in hAF co-cultured with macrophages but did not significantly influence the protein production of these enzymes. Our results findings suggest that notochordal cells might have a less supportive function for healing of annular degeneration than for NP degeneration.
In conclusion, we confirmed that macrophage-like cells lead to annular degeneration through the regulation of degradative enzymes and their inhibitors, produced by human AF cells, and rabbit notochordal cells could control the mRNA expression of these enzymes. Levels of MMP-3 and TIMP-1 showed parallel patterns during annular inflammation, while expressed from AF in an apparently contradictory pattern as counterpart enzymes after inflammation, suggesting that selective regulation of these enzymes may provide future therapeutic targets for treating symptomatic disc degeneration.
Acknowledgements
We thank Sung Shin Ahn, B.S., for their excellent technical assistance. This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2011-0021292) and supported by a Korea University Grant (K1132691). The manuscript submitted does not contain information about medical device(s)/drug(s). No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript. The authors declare that they have no competing interests. This work was approved by Korea University Guro Hospital Institutional Review Board and Korea University Institutional Animal Care and Use Committee.
References
1. Aguiar DJ, Johnson SL, Oegema TR. Notochordal cells interact with nucleus pulposus cells : regulation of proteoglycan synthesis. Exp Cell Res. 1999; 246:129–137. PMID: 9882522.

2. Andersson GB. Epidemiological features of chronic low-back pain. Lancet. 1999; 354:581–585. PMID: 10470716.

3. Bachmeier BE, Nerlich A, Mittermaier N, Weiler C, Lumenta C, Wuertz K, et al. Matrix metalloproteinase expression levels suggest distinct enzyme roles during lumbar disc herniation and degeneration. Eur Spine J. 2009; 18:1573–1586. PMID: 19466462.

4. Gomez-Camarillo MA, Almonte-Becerril M, Vasquez Tort M, Tapia-Ramirez J, Kouri Flores JB. Chondrocyte proliferation in a new culture system. Cell Prolif. 2009; 42:207–218. PMID: 19236380.

5. Kim JH, Deasy BM, Seo HY, Studer RK, Vo NV, Georgescu HI, et al. Differentiation of intervertebral notochordal cells through live automated cell imaging system in vitro. Spine (Phila Pa 1976). 2009; 34:2486–2493. PMID: 19841610.

6. Kim JH, Studer RK, Sowa GA, Vo NV, Kang JD. Activated macrophage-like THP-1 cells modulate anulus fibrosus cell production of inflammatory mediators in response to cytokines. Spine (Phila Pa 1976). 2008; 33:2253–2259. PMID: 18784630.

7. Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev. 2004; 84:649–698. PMID: 15044685.

8. Le Maitre CL, Freemont AJ, Hoyland JA. Localization of degradative enzymes and their inhibitors in the degenerate human intervertebral disc. J Pathol. 2004; 204:47–54. PMID: 15307137.

9. Masuda K. Biological repair of the degenerated intervertebral disc by the injection of growth factors. Eur Spine J. 2008; 17(Suppl 4):441–451. PMID: 19005698.

10. Masuda K, Miyabayashi T, Meachum SH, Eurell TE. Proliferation of canine intervertebral disk chondrocytes in three-dimensional alginate microsphere culture. J Vet Med Sci. 2002; 64:79–82. PMID: 11853153.

11. Masuhara K, Nakai T, Yamaguchi K, Yamasaki S, Sasaguri Y. Significant increases in serum and plasma concentrations of matrix metalloproteinases 3 and 9 in patients with rapidly destructive osteoarthritis of the hip. Arthritis Rheum. 2002; 46:2625–2631. PMID: 12384920.

12. Moon HJ, Joe H, Kwon TH, Choi HK, Park YK, Kim JH. Notochordal cells influence gene expression of inflammatory mediators of annulus fibrosus cells in proinflammatory cytokines stimulation. J Korean Neurosurg Soc. 2010; 48:1–7. PMID: 20717505.

13. Murphy G, Knäuper V, Atkinson S, Butler G, English W, Hutton M, et al. Matrix metalloproteinases in arthritic disease. Arthritis Res. 2002; 4(Suppl 3):S39–S49. PMID: 12110122.
14. Okuma M, Mochida J, Nishimura K, Sakabe K, Seiki K. Reinsertion of stimulated nucleus pulposus cells retards intervertebral disc degeneration : an in vitro and in vivo experimental study. J Orthop Res. 2000; 18:988–997. PMID: 11192261.

15. Peng B, Hao J, Hou S, Wu W, Jiang D, Fu X, et al. Possible pathogenesis of painful intervertebral disc degeneration. Spine (Phila Pa 1976). 2006; 31:560–566. PMID: 16508552.

16. Peng B, Wu W, Hou S, Li P, Zhang C, Yang Y. The pathogenesis of discogenic low back pain. J Bone Joint Surg Br. 2005; 87:62–67. PMID: 15686239.

17. Roberts S, Caterson B, Menage J, Evans EH, Jaffray DC, Eisenstein SM. Matrix metalloproteinases and aggrecanase : their role in disorders of the human intervertebral disc. Spine (Phila Pa 1976). 2000; 25:3005–3013. PMID: 11145811.
18. Roughley PJ. Biology of intervertebral disc aging and degeneration : involvement of the extracellular matrix. Spine (Phila Pa 1976). 2004; 29:2691–2699. PMID: 15564918.
19. Stetler-Stevenson WG, Seo DW. TIMP-2 : an endogenous inhibitor of angiogenesis. Trends Mol Med. 2005; 11:97–103. PMID: 15760767.
20. Tsuchiya S, Kobayashi Y, Goto Y, Okumura H, Nakae S, Konno T, et al. Induction of maturation in cultured human monocytic leukemia cells by a phorbol diester. Cancer Res. 1982; 42:1530–1536. PMID: 6949641.
21. Walmsley R. The development and growth of the intervertebral disc. Edinb Med J. 1953; 60:341–364. PMID: 13083579.
22. Weiler C, Nerlich AG, Zipperer J, Bachmeier BE, Boos N. 2002 SSE Award Competition in Basic Science : expression of major matrix metalloproteinases is associated with intervertebral disc degradation and resorption. Eur Spine J. 2002; 11:308–320. PMID: 12193991.

Fig. 1
Schematic representation of the in vitro co-culture experiment. hAF : human annulus fibrosus pellet (2×105/well), rNC : rabbit notochordal cell clusters (2×104/well), M : activated macrophage-like THP-1 cells (1×105/well), hAF(M) : previously macrophage-exposed hAF pellet, hAF(rNC-M) : previously rNC cell and macrophage exposed hAF pellets, PMA : 160 nM phorbol myristate acetate.
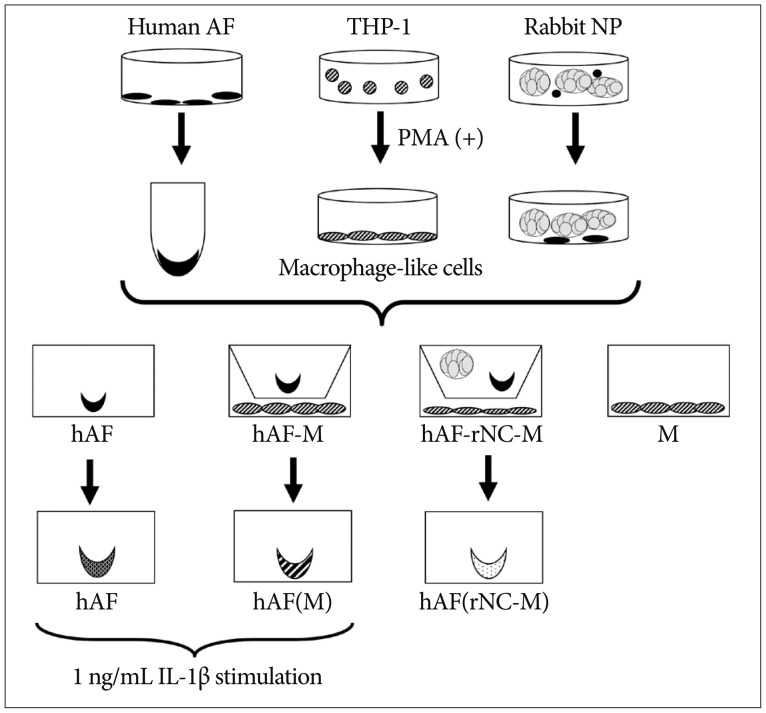
Fig. 2
MMPs, TIMPs, and IGF-1 production from co-cultured cells. The hAF pellet was co-cultured with macrophage-like cells for 48 h. The levels of MMP-1 (A), -3 (B), -9 (E), and TIMP-1 (C) were increased significantly in the conditioned media (CM) of hAF pellets co-cultured with macrophage-like cells (hAF-M) compared with hAF pellets alone (hAF), whereas the levels of TIMP-2 (D) and IGF-1 (F) were decreased. The data were presented as mean (five individual experiments, n=14-18 in each group, 95% confidence interval). MMPs : matrix metalloproteinases, TIMPs : tissue inhibitors of metalloproteinases, IGF-1 : insulin-like growth factor, hAF : human annulus fibrosus, M : macrophage-like cells.
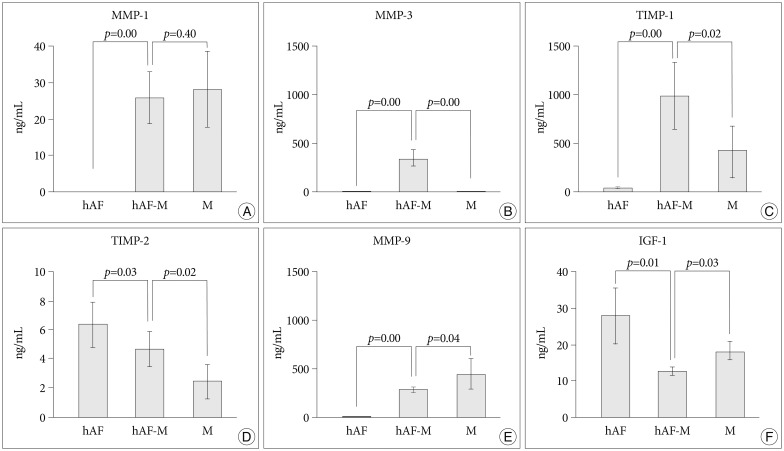
Fig. 3
Stimulation of nemotic hAF pellet by 1 ng/mL interleukin (IL)-1β. To evaluate the MMPs (A and B) and TIMPs (C and D) production levels in the hAF pellet with IL-1β stimulation, naïve hAF pellets (hAF) and nemotic hAF pellets (hAF(M)) were cultured with 1 ng/mL recombinant human IL-1β for 48 h. After macrophage exposure, hAF produced significantly higher levels of MMP-1 and -3 and lower levels of TIMP-1 and -2. Stimulation with 1 ng/mL IL-1β enhanced the levels of MMP-1 and -3 production significantly from hAF pellets and decreased the level of TIMP-2 significantly (p<0.05). The data were presented as mean (five individual experiments, n=14-18 in each group, 95% confidence interval). hAF : human annulus fibrosus, MMPs : matrix metalloproteinases, TIMPs : tissue inhibitors of metalloproteinases.

Fig. 4
hAF pellet expression of MMP-3 (A) and TIMP-1 (B) after co-culture. Gene expression was analyzed using real-time RT-PCR. MMP-3 expression was increased significantly by macrophage exposure (hAF(M)), and decreased significantly after co-culture with rNC cells and macrophages (hAF(rNC-M)) compared with hAF(M). TIMP-1 expression was decreased significantly in hAF(M) compared with hAF, and increased in hAF(rNC-M) compared with hAF(M). The data were presented as mean (three individual experiments, n=6-8 in each group, triplicated data, 95% confidence interval). hAF : human annulus fibrosus, MMPs : matrix metalloproteinases, TIMPs : tissue inhibitors of metalloproteinases, RT-PCR : reverse transcriptase polymerase chain reaction.

Fig. 5
MMPs (A and B) and TIMPs (C and D) production levels in hAF pellets after co-culture with rNC cells and macrophages. Co-culturing with rNC (hAF(rNC-M)) tended to decrease the levels of MMP-3 and TIMP-1, but did not affect the production of MMP-1 or TIMP-2 in the hAF pellet with macrophages (hAF(M)). The data were presented as mean (five different experiments, n=14-18 in each group, 95% confidence interval). MMPs : matrix metalloproteinases, TIMPs : tissue inhibitors of metalloproteinases, hAF : human annulus fibrosus.

Fig. 6
hAF pellet COL1A2 expression after co-culture. Gene expression was assessed by real-time RT-PCR. COL1A2 expression by hAF was not influenced by macrophage exposure (hAF(M)), 1 ng/mL interleukin (IL)-1β stimulation, or rNC cell treatment (hAF(rNC-M)) in 48-h cultures. The data were presented as mean (three individual experiments, n=6-8, triplicated data, 95% confidence interval). hAF : human annulus fibrosus, RT-PCR : reverse transcriptase polymerase chain reaction.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download