Abstract
Objective
The aim of this study was to evaluate the microanatomy and histological features of the central myelin in the root exit zone of facial nerve.
Methods
Forty facial nerves with brain stem were obtained from 20 formalin fixed cadavers. Among them 17 facial nerves were ruined during preparation and 23 root entry zone (REZ) of facial nerves could be examined. The length of medial REZ, from detach point of facial nerve at the brain stem to transitional area, and the thickness of glial membrane of central myelin was measured. We cut brain stem along the facial nerve and made a tissue block of facial nerve REZ. Each tissue block was embedded with paraffin and serially sectioned. Slices were stained with hematoxylin and eosin (H&E), periodic acid-Schiff, and glial fibrillary acid protein. Microscopy was used to measure the extent of central myelin and thickness of outer glial membrane of central myelin. Thickness of glial membrane was examined at two different points, the thickest area of proximal and distal REZ.
Results
Special stain with PAS and GFAP could be differentiated the central and peripheral myelin of facial nerve. The length of medial REZ was mean 2.6 mm (1.6-3.5 mm). The glial limiting membrane of brain stem is continued to the end of central myelin. We called it glial sheath of REZ. The thickness of glial sheath was mean 66.5 µm (40-110 µm) at proximal REZ and 7.4 µm (5-10 µm) at distal REZ.
Cranial nerves were composed of two different histological portions, glial and non-glial portion. The transitional zone refers to the junction between glial portion and the non-glial peripheral portion. The most outer layer of brain cortex is coated by pia glial membrane called glial limiting membrane or glial limitans10). The glial limiting membrane is extended to the transitional zone of cranial nerves. Skinner12) reported the extent of glial out growth into the various cranial nerves and glial out spread of facial nerve was extended as far as 2.6 mm in length from the origin of brain stem. In general, root exit zone or root entry zone (REZ) of cranial nerves means the area nerves come from the brainstem. Recently, some authors proposed to replace the term of REZ by root exit point (RExP), root detach point (RDP), and root exit zone (RExZ) in detail13). The purposes of this study were to measure the length of the central myelin, especially from root detach point to transitional zone and determine histological structures of central myelin of facial nerve.
Twenty brain stems were obtained from formalin embedded cadavers. The 12 men and 8 women of cadavers ranged in age from 32 to 78 years (mean, 58 years). During the brain stems were served, care should be taken to preserve the cisternal segment of facial nerve. We obtained 40 facial nerves but 17 facial nerves were put out of shape during preparation for histological study. Twenty three facial nerves could be examined properly. To make a facial nerve-brainstem tissue block, we cut the midline of brain stem into halves and slice them along the facial nerve. Each tissue block was embedded in paraffin and sectioned serially. First, one slice was stained with hematoxylin and eosin (H&E) for screening the shape of facial nerve and brain stem. And then, other slices were stained with periodic acid-Schiff (PAS) for peripheral myelin and glial fibrillary acid protein (GFAP) for central myelin and glial membrane. After staining, photomicrographs of each section were taken and measure the distance of medial REZ, from detach point of facial nerve at the brain stem to the most distal part of central myelin, and thickness of glial membrane of central myelin of REZ. The thickness of glial membrane was obtained at two different points, the thickest point of proximal and distal area of central myelin. Usually to correct for the shrinkage error during fixation, occulomotor nerve of same specimen was used as a standard. But we did not calculate the percentage of facial nerve shrinkage because of being used pre-fixed specimens with formalin.
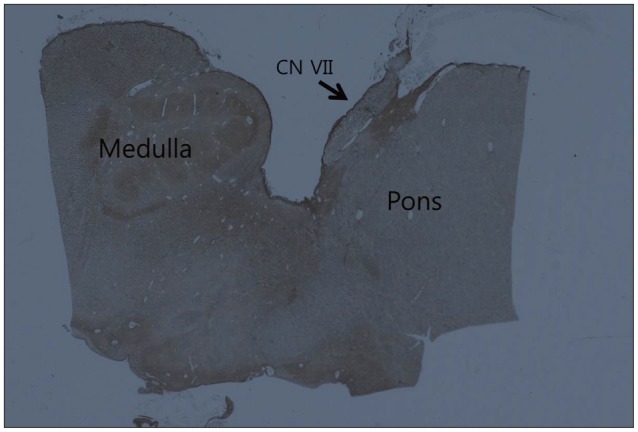
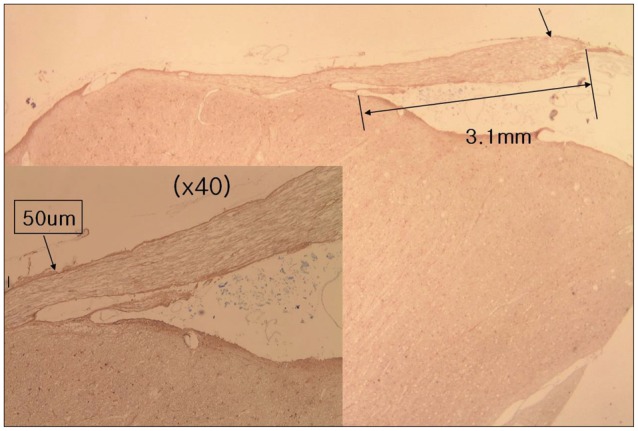
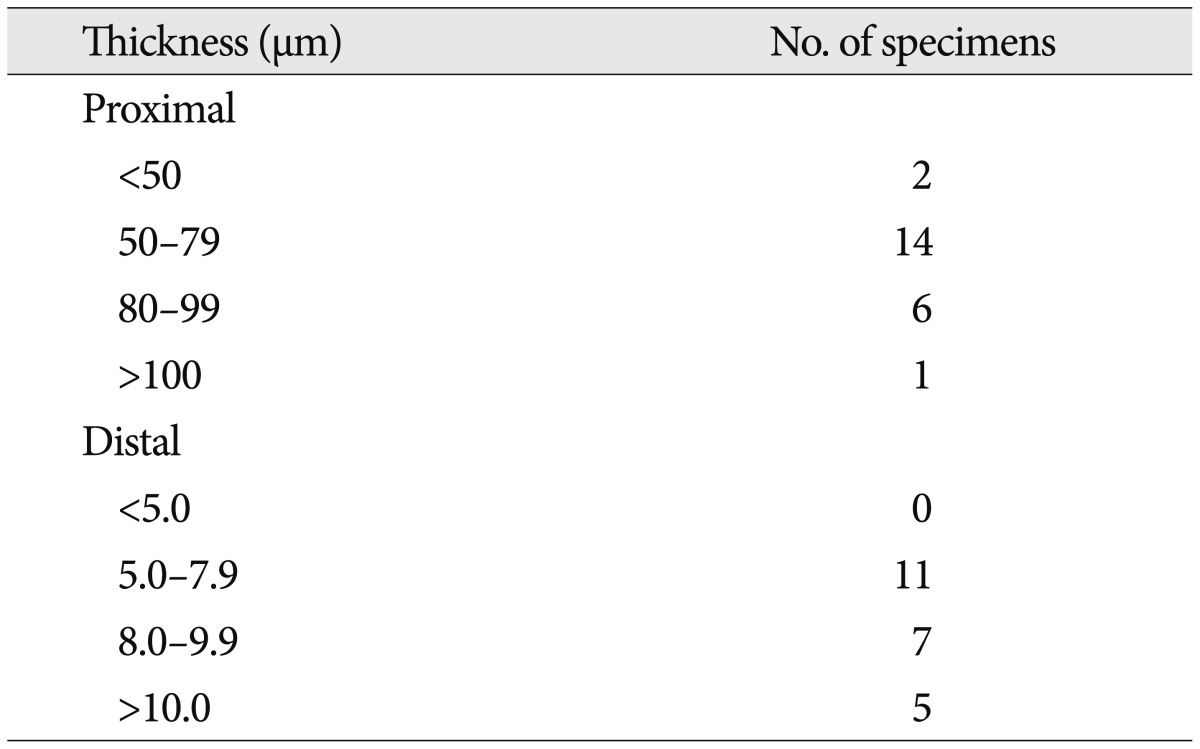
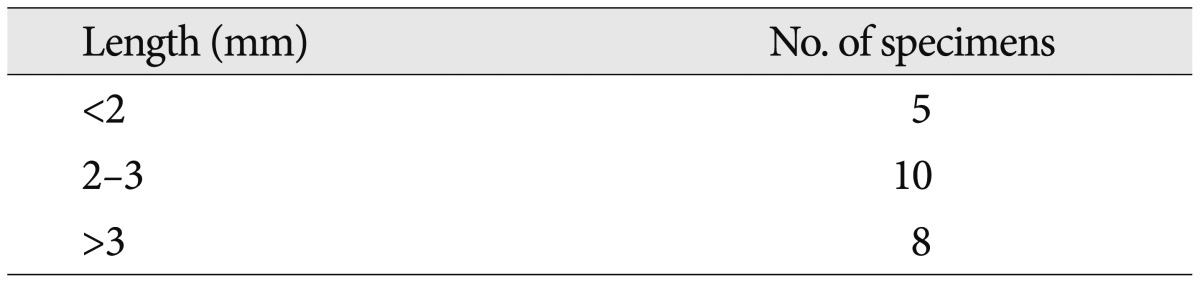
With the H&E staining, the shape of facial nerve was easily determined (Fig. 1). And special staining methods, such as PAS and GFAP, could be clearly distinguished the facial nerve into peripheral and central myelin. Peripheral myelin was stained dark blue with PAS staining and central myelin was stained dark brown with GFAP (Fig. 2). Glial outgrowth from the brain stem, central myelin, ends in dome or cone formation. The outermost membrane surrounding the brain stem, glial limiting membrane, continues to the central myelin could be visible. And its thickness tends to decrease gradually (Fig. 3). The mean thickness of glial membrane of central myelin was 66.5 um (40-110 um) at proximal and 7.4 um (range of 5-10 um) at distal (Table 1). The length of medial REZ was mean 2.6 mm (range of 1.6-3.5 mm) (Table 2).
There is no clear description of the relationship between the REZ and the transitional zone. In the strict sense, REZ means the area nerve comes from the brainstem and transitional zone refers to glia-schwann cell border. The term of REZ was defined by Jannetta as a junctional area between central and peripheral myelin6). Many authors used the terms of REZ and transitional zone interchangeably1,3,11). In a recent study, Tomii et al.13) described the transitional zone as the region where the myelin sheath is composed of both central glial and schwann cell myelin. They have proposed the use of terms root exit point (RExP), RDP (medial detach point) and transitional zone instead of REZ. And they stressed the RDP appears to be a good landmark for the microvascular decompression surgery.
In 1915, Henschen5) has been descried that cranial nerve was composed of two different segments, such as glial and non-glial portion. Skinner12) also reported several characteristics of various cranial nerves as follows. From the brain there is an outgrowth of glia cells, astrocyte and oligodendroglia, along the cranial nerve trunks and the extent of glial outgrowth varies in different nerves. Many investigators named the glial outgrowth segment of cranial nerve as CNS segment of cranial nerve, central myelin, or central glial myelin2,10,13). To measure the exact length of central myelin of facial nerve is difficult because of its irregular distal margin. Skinner12) reported that the glial outspread in the facial nerve extended as far as 2.6 mm from the plane of superficial origin. Adams1) described that the transitional zone is only 1 to 3 mm in length. Jannetta6) also reported the distance to the peripheral boundary of the REZ was 0.5 to 1 cm in the fifth, seventh, and ninth cranial nerves. In 2003, Tomii13) described the root exit zone of facial nerve more detail. He measured the length of the lateral and medial transitional zone were mean 1.9 mm and 0.58 mm, respectively. We measured the distance from medial detach point of brain stem to the most distal glial segment of facial nerve.
Glial portion of the cranial nerve, central myelin, is composed of fibrillary astrocyte and oligodendroglia cells12). And it has a structure similar to that of white matter of the CNS, consisting of parallel traveling nerve fibers, and lacks funicular structure. Nerve fibers are embedded in supporting tissue, but endo-, peri, and epineurium are absent. De Ridder et al.2) stated the layer of pia mater surrounds the central myelin. Other reported central myelin is lined with astrocyte foot plates8). Glial limiting membrane, pia glial membrane, or glial limitans, is a thin barrier of astrocyte foot process associated with the parenchymal basal lamina surrounding the brain10). The main function of glial limiting membrane is to act as a physical barrier of the CNS. We observed that the glial limiting membrane extending from the outermost layer of brain stem, strong stained with GFAP, surrounds the central myelin. We called the layer closing the central myelin as glial sheath like epineural sheath of peripheral nerve. We also measured the thickness of glial sheath of facial nerve and it becomes thin at distal portion than proximal. The glial sheath of central myelin may act as a barrier of the nerve fiber from vascular compression. The cause of hemifacial spams is generally known as that facial nerve compressed by blood vessels located at the REZ. The mechanism of hemifacial spasm is thought that thinning or defect of neural sheath of facial nerve because of vascular compression may result in developing cross talking of neural transmission4,9). Jannetta et al.7) reported that myelin defect exist in the REZ in patient of hemifacial spasm.
Our study shows the length of medial REZ is mean 2.6 mm (range of 1.6-3.5 mm) and the REZ is covered by glial sheath continued from glial limiting membrane of brain stem. Glial sheath of central myelin tends to become thin toward transitional zone. Further studies are needed to ascertain the function of glial sheath as a barrier.
References
1. Adams CB. Microvascular compression : an alternative view and hypothesis. J Neurosurg. 1989; 70:1–12. PMID: 2642544.
2. De Ridder D, Møller A, Verlooy J, Cornelissen M, De Ridder L. Is the root entry/exit zone important in microvascular compression syndromes? Neurosurgery. 2002; 51:427–433. discussion 433-434. PMID: 12182781.

3. Gardner WJ. Concerning the mechanism of trigeminal neuralgia and hemifacial spasm. J Neurosurg. 1962; 19:947–958. PMID: 13946557.

4. Gardner WJ. Cross talk-the paradoxical transmission of a nerve impulse. Arch Neurol. 1966; 14:149–156. PMID: 4378157.

5. Henschen F. Zur Histologie und Pathogenese der Kleinhirnbrückenwinkeltumoren. Arch Psychiat Nervenkr. 1915; 56:21–122.

6. Jannetta PJ. The cause of hemifacial spasm : definitive microsurgical treatment at the brainstem in 31 patients. Trans Sect Otolaryngol Am Acad Ophthalmol Otolaryngol. 1975; 80(3 Pt 1):319–322.
7. Jannetta PJ, Abbasy M, Maroon JC, Ramos FM, Albin MS. Etiology and definitive microsurgical treatment of hemifacial spasm. Operative techniques and results in 47 patients. J Neurosurg. 1977; 47:321–328. PMID: 894338.
8. McFarland DE, Friede RL. Number of fibres per sheath cell and internodal length in cat cranial nerves. J Anat. 1971; 109(Pt 1):169–176. PMID: 5556669.
9. Møller AR, Jannetta PJ. On the origin of synkinesis in hemifacial spasm results of intracranial recordings. J Neurosurg. 1984; 61:569–576. PMID: 6086858.

10. Nakazawa E, Ishikawa H. Ultrastructural observations of astrocyte end-feet in the rat central nervous system. J Neurocytol. 1998; 27:431–440. PMID: 10192524.
11. Peker S, Kurtkaya O, Uzün I, Pamir MN. Microanatomy of the central myelin-peripheral myelin transition zone of the trigeminal nerve. Neurosurgery. 2006; 59:354–359. discussion 354-359. PMID: 16883175.

12. Skinner HA. Some histologic features of the cranial nerves. Arch Neurol Psychiatry. 1931; 25:356–372.

13. Tomii M, Onoue H, Yasue M, Tokudome S, Abe T. Microscopic measurement of the facial nerve root exit zone from central glial myelin to peripheral Schwann cell myelin. J Neurosurg. 2003; 99:121–124. PMID: 12854753.

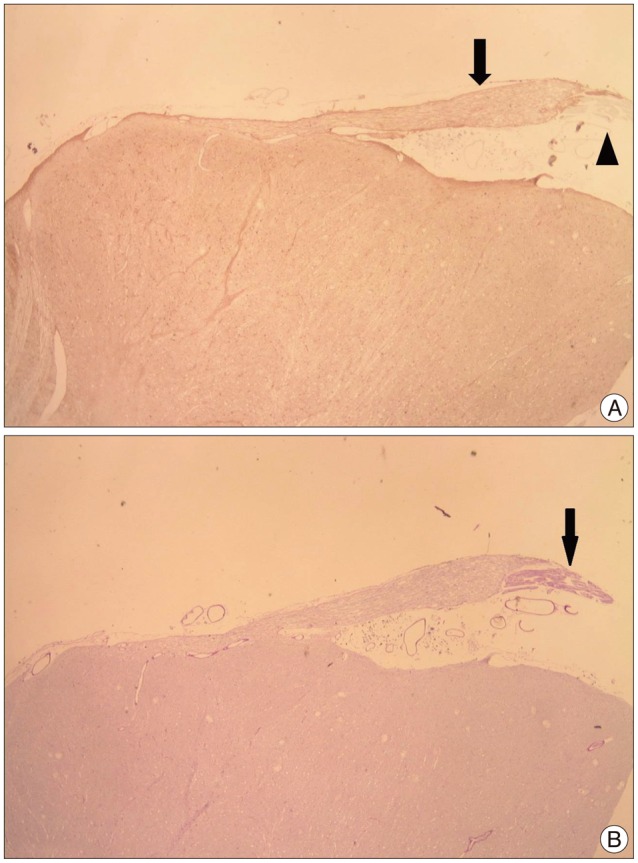
Fig. 2
Photomicrograph showing an exiting facial nerve from brain stem. A : Central segment of facial nerve positive to glial fibrillary acid protein (arrow) turned out to be a brown color and peripheral segment is not stained (arrowhead). B : Peripheral segment of facial nerve positive to periodic acid-Schiff (arrow) turned out to be a deep purple color (original magnification, ×10).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download