Abstract
We describe a rare case of intradural-extramedullary primary spinal cysticercosis. A 42-year-old man visited our institute for lower back pain. He denied having consumed raw meet. Magnetic resonance (MR) images revealed an intradural pure cystic mass at the L3-L4 level. A radiologic diagnosis of spinal arachnoid cyst was established. Three years later, he complained of aggravated back pain, and follow-up MR examination showed a markedly expanded cyst, occupying the subarachnoid space from the T11 to the S1 level. L2 hemilaminectomy was performed, and a yellowish infected cyst bulged out through the dural opening. The cyst was removed en bloc. The histopathological findings of the cyst were consistent with parasitic infection. Serum enzyme-linked immunosorbent assay (ELISA) confirmed the presence of spinal cysticercosis. As there was no intracranial lesion, the final diagnosis was primary spinal cysticercosis, which is very rare. MR imaging is a sensitive diagnostic tool for detecting cystic lesions in the spine; however, it is difficult to distinguish cysticercosis from non-infectious cysts such as an arachnoid cyst without using gadolinium enhancement. Clinicians treating spinal cysts with an unusual clinical course should include cysticercosis as a differential diagnosis. We recommend contrast-enhanced MR imaging and serum ELISA in the diagnostic work-up of such cases.
Neurocysticercosis can be categorized into parenchymal, leptomeningeal, intraventricular, or spinal cysticercosis according to the location of the lesions7). Spinal cysticercosis accounts for only 1-3% of all cases of neurocysticercosis, and the majority of spinal lesions are accompanied by some intracranial involvement12). About 80% of spinal cysticercosis are intradural-extramedullary (IDEM) forms3,4), which are difficult to distinguish from arachnoid cysts. The pathogenesis of spinal cysticercosis involves the migration of the cysticerci through the ventricular system (Table 1). A diagnosis of primary spinal cysticercosis (PSC) indicates neurocysticercosis of the spinal cord or of the spinal perimeningeal structures, without any concurrent intracranial involvement. To the best of our knowledge, there are only 6 reported cases of IDEM-PSC in the English literature (Table 2)4,6,7,11,12,14). This report describes our experience of a rare case of IDEM-PSC that was misdiagnosed as an arachnoid cyst.
A 42-year-old man visited out institute for back pain. He denied having eaten any raw meet, including pork. Magnetic resonance imaging (MRI) of the lumbar spine revealed an intradural cystic lesion at the L3-4 level. The lesion was homogeneous in terms of signal intensities, and was isointense on a T1-weighted image, and hyper-intense on a T2-weighted image, indicating that it contained cerebrospinal fluid (CSF). Initially, intradural arachnoid cyst was suspected based on radiologic study results and conservative treatment was decided. Three years later, he complained of aggravated back pain. A follow-up MRI examination revealed that the cyst had expanded from the T11 to the S1 (Fig. 1).
We planned to fenestrate the wall of the cyst, and performed an L2 right hemi-laminectomy. A yellowish cystic lesion was bulged out through the opening of the durotomy. The cyst was easily removed by gentle traction of the cyst wall (Fig. 2) and complete removal of the cyst was confirmed by postoperative MRI (Fig. 1). Histopathological examination revealed a translucent cyst wall with eosinophilic lining and chronic inflammatory cells, which is consistent with parasitic infection (Fig. 3). Serum enzyme-linked immunosorbent assay (ELISA) was positive for cysticercosis. The final diagnosis was primary spinal cysticercosis, as his brain MRI demonstrated an absence of intracranial lesions. The patient was subsequently treated with oral albendazole (15 mg/kg/day) for 30 days, to prevent recurrence. He recovered uneventfully and experienced no back pain after the surgery.
It is difficult to differentiate IDEM spinal cysticercosis from intradural cystic lesions by use of MRI. In fact, among the 7 cases of PSC reported to date (Table 2), including the one reported here, only 1 case, who had been treated previously for spinal neurocysticercosis, was diagnosed preoperatively as having spinal neurocysticercosis6). The detection of an intradural cystic lesion through MRI leads primarily to a differential diagnosis of arachnoid cyst versus spinal cysticercosis, based on incidence. Moreover, other lesions, such as dermoid cysts, hydatid cysts, tuberculosis, sarcoidosis, and forms of subarachnoid neoplasia, have to be taken into consideration in the differential diagnosis of the disease12). Although arachnoid cysts and spinal cysticercosis have similar features on MRI, their treatment is different. Thus, the differential diagnosis of these conditions is important and requires other ancillary diagnostic measures. CSF examination in patients with neurocysticercosis usually reveals moderate lymphocytic pleocytosis, variable eosinophilic pleocytosis, elevated protein levels, and low glucose levels. However, peripheral blood smears, eosinophilia, and CSF assessment are neither sensitive nor specific1). Serum or CSF ELISA is a confirmative diagnostic tool for neurocysticercosis in cases in which MRI findings are not definitive. Nevertheless, the authors did not perform an ELISA examination before the surgery because the patient denied having eaten raw pork. The serum ELISA for cysticercosis performed after the surgery was positive. It may be reasonable to include serum ELISA in the differential diagnosis of spinal intradural cystic lesions, particularly in cysticercosis endemic regions such as Korea5).
MRI is a sensitive diagnostic tool for the identification of cystic lesions in the spine; however, it is difficult to distinguish cysticercosis from a noninfectious cyst, such as an arachnoid cyst, without using gadolinium enhancement. Contrast-enhanced MRI is mandatory for evaluating spinal cysticercosis13). The wall of the arachnoid cyst does not appear enhanced, whereas that of the cysticerci are well enhanced in gadolinium enhanced MR images10). The cyst walls were enhanced in all reported PSC cases. Moreover, the MRI sequence can distinguish between the CSF like fluid of the arachnoid cyst and the protein-rich fluid of an infectious cyst2). Because arachnoid cysts have a low concentration of metabolites, similar to the CSF, the signal intensity is not increased relative to that of the CSF. On T2-weighted MR images, the signal intensity of neurocysticercosis is increased because of its higher protein content. Our experience with this case and our review of the published literature suggest that contrast-enhanced MRI may provide useful information in the differential diagnosis of spinal cystic lesions, especially in patients with an unusual clinical course, such as the present case.
Although MRI is the method of choice for the diagnosis of neurocysticercosis, particularly in the presence of calcification, computed tomography (CT) has an advantage compared with MRI12). The initial stage of cysticercosis is characterized by less tissue response. At this stage, CT shows areas of decreased density surrounding the lesion and does not have an advantage compared with MRI as a diagnostic method. At the final stage of the disease, the dead larvae have calcified, and CT shows multiple calcified nodules, which are best identified on CT compared with MRI5).
The surgical excision of intramedullary spinal cysticercosis may carry some risk of neurological insult. Regarding the intramedullary form, some authors have reported a successful outcome using steroid and albendazole therapy, without surgical excision1,9). However, it seems that surgical intervention is mandatory for IDEM spinal cysticercosis. The excision of an extramedullary lesion does not always lead to neurological deficits, and patients present with progressive neurological dysfunctions, regardless of whether any medical treatment has been attempted. Therefore, in cases of IDEM spinal cysticercosis, surgical treatment is necessary, both to provide a definitive diagnosis and to alleviate the compressive symptoms immediately.
In this case, we used only one level hemilaminectomy in the center of the lesion site. The spinal cysticerci had well-defined margins and were easily excised without remnants through the small window. We propose that limited laminectomy can be sufficient for the surgical excision of IDEM spinal cysticercosis.
Spinal cysticercosis can be easily misdiagnosed as an arachnoid cyst, and these lesions are usually indistinguishable without ELISA and contrast-enhanced MRI. When dealing with patients with spinal intradural cysts showing an unusual clinical course, clinician should consider performing enhanced MRI and ELISA for differential diagnosis.
References
1. Azfar SF, Kirmani S, Badar F, Ahmad I. Isolated intramedullary spinal cysticercosis in a 10-year-old female showing dramatic response with albendazole. J Pediatr Neurosci. 2011; 6:52–54. PMID: 21977090.
2. Bustos JA, Pretell EJ, Llanos-Zavalaga F, Gilman RH, Del Brutto OH, Garcia HH, et al. Efficacy of a 3-day course of albendazole treatment in patients with a single neurocysticercosis cyst. Clin Neurol Neurosurg. 2006; 108:193–194. PMID: 16412842.

3. De Souza Queiroz L, Filho AP, Callegaro D, De Faria LL. Intramedullary cysticercosis. Case report, literature review and comments on pathogenesis. J Neurol Sci. 1975; 26:61–70. PMID: 1159459.
4. Gupta S, Singh PK, Gupta B, Singh V, Azam A. Isolated primary intradural extramedullary spinal neurocysticercosis: a case report and review of literature. Acta Neurol Taiwan. 2009; 18:187–192. PMID: 19960962.
5. Hawk MW, Shahlaie K, Kim KD, Theis JH. Neurocysticercosis : a review. Surg Neurol. 2005; 63:123–132. discussion 132. PMID: 15680651.
6. Jang JW, Lee JK, Lee JH, Seo BR, Kim SH. Recurrent primary spinal subarachnoid neurocysticercosis. Spine (Phila Pa 1976). 35:2010; E172–E175. PMID: 20118838.

7. Jongwutiwes U, Yanagida T, Ito A, Kline SE. Isolated intradural-extramedullary spinal cysticercosis : a case report. J Travel Med. 2011; 18:284–287. PMID: 21722242.

8. Leite CC, Jinkins JR, Escobar BE, Magalhães AC, Gomes GC, Dib G, et al. MR imaging of intramedullary and intradural-extramedullary spinal cysticercosis. AJR Am J Roentgenol. 1997; 169:1713–1717. PMID: 9393195.

9. Mohanty A, Venkatrama SK, Das S, Das BS, Rao BR, Vasudev MK. Spinal intramedullary cysticercosis. Neurosurgery. 1997; 40:82–87. PMID: 8971828.

10. Nash TE, Neva FA. Recent advances in the diagnosis and treatment of cerebral cysticercosis. N Engl J Med. 1984; 311:1492–1496. PMID: 6390196.

11. Park YS, Lee JK, Kim JH, Park KC. Cysticercosis of lumbar spine, mimicking spinal subarachnoid tumor. Spine J. 2011; 11:e1–e5. PMID: 21474075.

12. Paterakis KN, Kapsalaki E, Hadjigeorgiou GM, Barbanis S, Fezoulidis I, Kourtopoulos H. Primary spinal intradural extramedullary cysticercosis. Surg Neurol. 2007; 68:309–311. discussion 312. PMID: 17719976.

13. Rahalkar MD, Shetty DD, Kelkar AB, Kelkar AA, Kinare AS, Ambardekar ST. The many faces of cysticercosis. Clin Radiol. 2000; 55:668–674. PMID: 10988043.

14. Shin SH, Hwang BW, Lee SJ, Lee SH. Primary extensive spinal subarachnoid cysticercosis. Spine (Phila Pa 1976). 37:2012; E1221–E1224. PMID: 22588382.

Fig. 1
Magnetic resonance imaging of the lumbar spine : Sagittal T2-weighted image showing an intradural cystic lesion (arrow) that is well circumscribed at the L3-L4 level (A). Three years later, follow-up sagittal (B) and axial (C) T2-weighted images of the L2 level reveal that the cyst (arrow) has grown, and occupies the T11-S1 levels. Post-operative images showing that the spinal cysticercosis has been removed without remnants (D).
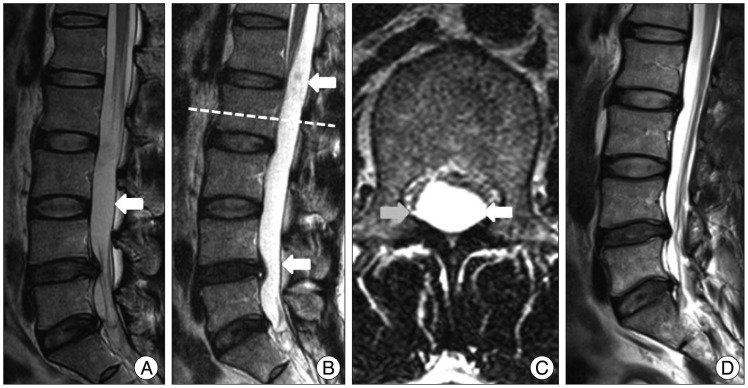
Fig. 2
Intraoperative photographs showing the cystic lesion observed after opening the dura (asterisk). The removed cyst was an oval-shaped yellowish lesion.
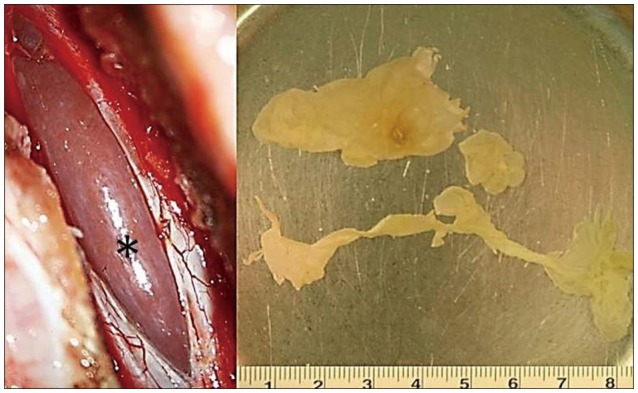
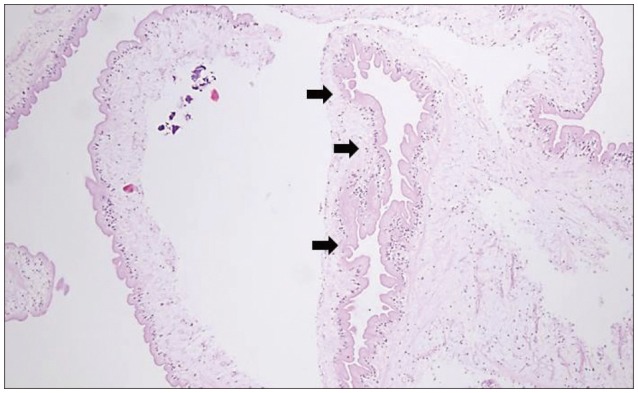
Fig. 3
Photomicrograph of the specimen showing a translucent cyst wall with eosinophilic lining (black arrows), and chronic inflammatory cells (Hematoxylin & Eosin, original magnification ×100).




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download