Abstract
Recently, the increasing rates of facial nerve preservation after vestibular schwannoma (VS) surgery have been achieved. However, the management of a partially or completely damaged facial nerve remains an important issue. The authors report a patient who was had a good recovery after a facial nerve reconstruction using fibrin glue-coated collagen fleece for a totally transected facial nerve during VS surgery. And, we verifed the anatomical preservation and functional outcome of the facial nerve with postoperative diffusion tensor (DT) imaging facial nerve tractography, electroneurography (ENoG) and House-Brackmann (HB) grade. DT imaging tractography at the 3rd postoperative day revealed preservation of facial nerve. And facial nerve degeneration ratio was 94.1% at 7th postoperative day ENoG. At postoperative 3 months and 1 year follow-up examination with DT imaging facial nerve tractography and ENoG, good results for facial nerve function were observed.
The vestibular schwannoma (VS) that surrounds the vestibular nerve is a benign tumor originating from Schwann cells. Postoperative facial nerve weakness, however, is reported in 8% to 20% of patients in the immediate postoperative period7). The incidence is as high as 25% of patients when delayed postoperative paralysis is considered10). The incidence of facial nerve dysfunction after VS resection has significantly decreased with the widespread use of microsurgical techniques. Increasing expectations have paralleled improvements in techniques and technologies, and the introduction of intraoperative facial nerve monitoring19,21). Despite these advancements, the most troubling postoperative impairment experienced by many patients is facial nerve dysfunction, which affects patients more substantially than physicians might predict4,11).
Diffusion tensor (DT) imaging of the facial nerve tractography-based fiber tracking is an established imaging technique that allows for the 3-dimentional reconstruction of white matter fibers, such as the pyramidal tract14,15,16). Its feasibility to show the position of cranial nerves in the cerebellopontine angle (CPA) has been demonstrated recently in individual patients with VS3). In this report, we describe a successful case that had a preservation of the facial nerve function after a nerve repair using glue-coated collagen fleece (Tachocomb®) during VS surgery. We were able to verify the anatomical preservation and functional outcome of the facial nerve with postoperative DT imaging facial nerve tractography, electroneurography (ENoG) and House-Brackmann (HB) grade9).
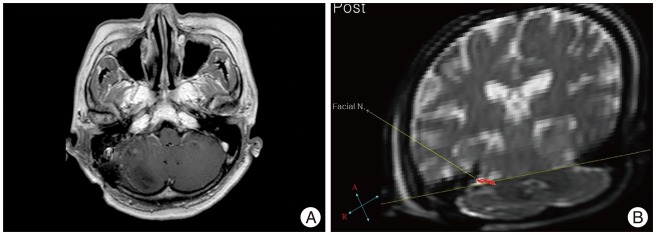
A 74-year-old female presented with a 7-year history of right-sided tinnitus and hearing loss without facial paresis in a hospital. A neurological examination of the patient on admission was nonspecific. A VS in the right CPA was seen on a magnetic resonance (MR) image (Fig. 1A). The lesion significantly compressed the brainstem. Preoperative DT imaging facial nerve tractography was performed to visualize the course of the facial nerve which is displaced anteriorly by VS (Fig. 1B).
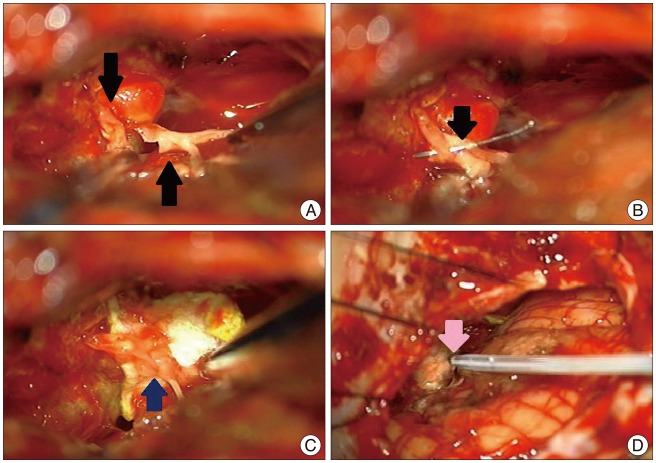
We performed a tumor resection through the retromastoid approach with intraoperative neuro-monitoring as brainstem auditory evoked potential (BAEP), electromyography (EMG) and nerve conduction study (NCS). The internal decompression of the tumor and careful dissection of surrounding neural tissue including the facial nerve were performed under intraoperative monitoring. In this process, the facial nerve was cut. During surgery, we found that the facial nerve was thin and fragile because of severe indentation by the tumor mass (Fig. 2A). First, we tried to do a primary end to end anastomosis but failed due to a thin and weak facial nerve (Fig. 2B). Glue-coated collagen fleece was then used to connect the facial nerve (Fig. 2C, D).
The patient presented, in the immediate postoperative period, with a significant facial palsy. Complete right eye closure was not possible. A shape of the mouth was asymmetrical with maximal effort (HB grade V). Postoperative MR images showed no residual tumor (Fig 3A). We checked postoperative DT imaging tractography for anatomical preservation of facial nerve and ENoG for prediction of functional outcome of repaired facial nerve. In postoperative ENoG (7th postoperative days), the facial nerve degeneration ratio was 94.1%. In addition, a postoperative DT imaging facial nerve tractography visualized the pathway of the facial nerve which was connected well (3rd postoperative days) (Fig. 3B). And good results for facial nerve function were observed on additional follow-up examination.
The patient was referred for intensive physiotherapy treatment, including participation in a facial nerve training program and periodical clinical examinations. One year later, the patient under-went a follow-up examination at our outpatient department. Follow-up DT imaging facial nerve tractography had no interval change as compared with the immediate postoperative study. A follow-up ENoG revealed that the facial nerve degeneration ratio had not chang-ed compared to an immediate postoperative ENoG. The patient's facial function had improved markedly (HB grade II). No synkinesis was observed by the patient or identified on physical examination.
Postoperative facial nerve injury is one of the major complications of a VS resection surgery. There is a disruption of the facial nerve in an estimated 2% to 10% of VS resections2). Satisfactory facial nerve function has important implications for a patient's quality of life after VS surgery.
The reliable preoperative visualization of the facial nerve location in relation to the VS would allow surgeons to plan tumor removal accordingly and may increase the safety of surgery6). Recent MR imaging modalities, such as diffusion-weighted imaging and DT imaging tractography, have allowed mapping of the cranial nerves in healthy individuals8). DT imaging tractography is a novel modality of MR imaging analysis that measures the diffusion direction of water molecules by combining multiple diffusion-weighted image scans taken from multiple gradient directions. The diffusion of water molecules is thought to be anisotropic inside white matter tracts12,13) and therefore maximal along the direction of the fiber tracts12). A 3D vector field (tensor) is assigned to each voxel. This information is then used to reconstruct and represent pictorially the white matter tracts within specific regions of interest. DT imaging tractography reconstruction was considered successful if a continuous tract of fibers was seen to extend from the internal acoustic meatus to the brainstem along the tumor capsule17). Taoka et al.20) applied these DT imaging tractography techniques to patients with VSs, and the authors reported that they were able to identify the location of the facial nerve in a subset of patients. We therefore consider the technique of DT imaging tractography to be a powerful tool for preoperatively predicting the course of the displaced facial nerve in VS. This information may increase the safety of surgery by enabling preservation of facial nerve function21).
With recent progress in surgical techniques for resection surgery of VS resulting in diminished morbidity and mortality, the expectation for the preservation of facial nerve function after surgery has increased1). Nerve preservation is the most obvious course in achieving satisfactory function, but nerve reconstruction and nerve reanimation may also be necessary to achieve acceptable results18).
Occasionally, the facial nerve can be transected during tumor resection. Immediate repair of an intentionally or unintentionally transected facial nerve is recommended to restore function. Different technical solutions are available in the case of facial nerve interruption, but it has been determined that, when feasible, end-to-end reapproximation represents the best option. In cases with longer nerve defects, not favorable for tensionless end-to-end anastomosis, a facial nerve interposition graft is indicated for bridging the defect. But the proximal facial nerve stump at the brainstem is either too short or impossible to identify, an intracranial cable nerve interposition grafting cannot be accomplished. In these situations, other reconstructive techniques, such as hypoglossal-facial nerve anastomosis, neuromuscular free flaps, and cross-face nerve grafting, are effective alternatives.
Bacciu et al.2) reported a study that compared the facial nerve outcomes of patients who underwent facial nerve cable grafting by using fibrin glue with those of patients who underwent facial nerve cable grafting by using microsuture. Above study, the functional results after facial nerve cable grafting by using fibrin glue exclusively were equivalent to those obtained with microsuture. Several advantages of a sutureless anastomosis such as less foreign body reaction, less trauma to the nerve from the suture, limitation of scar in the anastomosis, have been recognized. Also, the major advantages of the method of facial nerve grafting using fibrin glue are the simplicity, safety, and speed of this technique.
Our case is similar to the above study. We obtained good results without microsuture. We used only the end-to-end reapproximation method without cable graft. Furthermore, we could confirm anatomical preservation of the repaired facial nerve by postoperative DT imaging tractography, and could predict the excellent functional outcome of the repaired facial nerve by postoperative ENoG. The degeneration ratio was 94.1% in postoperative ENoG. Moreover, the postoperative DT imaging facial nerve tractography MR imaging demonstrated that the facial nerve was well preserved. We speculated that the fiber of nerve end was alive and the function of nerve signal transmission was partially preservation by end-to-end attachment using glue-coated collagen fleece although facial nerve was totally transected during the operation.
Electromyography may assist in prognosticating a functional return, determining neural conduction across the site of injury and following reinnervation in the recovery period. In a patient with Bell's palsy, a facial nerve degeneration ratio of less than 98% in ENoG is reported to have a good recovery5). Therefore, we expected that she would have good prognosis of facial nerve function because the facial nerve degeneration ratio was 94.1% in postoperative ENoG.
The patient was referred to intensive physiotherapy treatment. At postoperative 1 year follow-up a DT imaging facial nerve tractography and follow-up ENoG had not interval change as compared with the immediate postoperative study. However, the patient's facial function had improved to HB grade II.
The present case shows that end-to-end reapproximation methods could produce good results for the treatment of the injured facial nerve during tumor resection. This procedure also has a likelihood of functional improvement of repaired facial nerve. Therefore, we think that the facial nerve repair technique using glue-coated collagen fleece is a possible alternative method to primary end-to-end anastomosis. This process can be easily and quickly done and may have promising results with low morbidity. The authors report for the first time that anatomical preservation of the repaired facial nerve by postoperative DT imaging tractography, with the prediction of improvement of facial nerve function through postoperative ENoG.
References
1. Anderson DE, Leonetti J, Wind JJ, Cribari D, Fahey K. Resection of large vestibular schwannomas : facial nerve preservation in the context of surgical approach and patient-assessed outcome. J Neurosurg. 2005; 102:643–649. PMID: 15871506.

2. Bacciu A, Falcioni M, Pasanisi E, Di Lella F, Lauda L, Flanagan S, et al. Intracranial facial nerve grafting after removal of vestibular schwannoma. Am J Otolaryngol. 2009; 30:83–88. PMID: 19239948.

3. Chen DQ, Quan J, Guha A, Tymianski M, Mikulis D, Hodaie M. Three-dimensional in vivo modeling of vestibular schwannomas and surrounding cranial nerves with diffusion imaging tractography. Neurosurgery. 2011; 68:1077–1083. PMID: 21242825.

4. Cross T, Sheard CE, Garrud P, Nikolopoulos TP, O'Donoghue GM. Impact of facial paralysis on patients with acoustic neuroma. Laryngoscope. 2000; 110:1539–1542. PMID: 10983957.

5. Dumitru D, Walsh NE, Porter LD. Electrophysiologic evaluation of the facial nerve in Bell's palsy. A review. Am J Phys Med Rehabil. 1988; 67:137–144. PMID: 3041998.
6. Gerganov VM, Giordano M, Samii M, Samii A. Diffusion tensor imaging-based fiber tracking for prediction of the position of the facial nerve in relation to large vestibular schwannomas. J Neurosurg. 2011; 115:1087–1093. PMID: 21962081.

7. Hastan D, Vandenbroucke JP, van der Mey AG. A meta-analysis of surgical treatment for vestibular schwannoma : is hospital volume related to preservation of facial function? Otol Neurotol. 2009; 30:975–980. PMID: 19668100.
8. Hodaie M, Quan J, Chen DQ. In vivo visualization of cranial nerve pathways in humans using diffusion-based tractography. Neurosurgery. 2010; 66:788–795. discussion 795-796. PMID: 20305498.

9. House JW, Brackmann DE. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985; 93:146–147. PMID: 3921901.

10. Magliulo G, D'Amico R, Di Cello P. Delayed facial palsy after vestibular schwannoma resection : clinical data and prognosis. J Otolaryngol. 2003; 32:400–404. PMID: 14967087.

11. Magliulo G, Zardo F, Damico R, Varacalli S, Forino M. Acoustic neuroma : postoperative quality of life. J Otolaryngol. 2000; 29:344–347. PMID: 11770141.
12. Mori S, van Zijl PC. Fiber tracking : principles and strategies - a technical review. NMR Biomed. 2002; 15:468–480. PMID: 12489096.
13. Mukherjee P, Chung SW, Berman JI, Hess CP, Henry RG. Diffusion tensor MR imaging and fiber tractography : technical considerations. AJNR Am J Neuroradiol. 2008; 29:843–852. PMID: 18339719.

14. Nimsky C, Ganslandt O, Buchfelder M, Fahlbusch R. Intraoperative visualization for resection of gliomas : the role of functional neuronavigation and intraoperative 1.5 T MRI. Neurol Res. 2006; 28:482–487. PMID: 16808876.

15. Nimsky C, Ganslandt O, Fahlbusch R. Implementation of fiber tract navigation. Neurosurgery. 2007; 61(1 Suppl):306–317. discussion 317-318. PMID: 18813159.

16. Nimsky C, Ganslandt O, Hastreiter P, Wang R, Benner T, Sorensen AG, et al. Preoperative and intraoperative diffusion tensor imaging-based fiber tracking in glioma surgery. Neurosurgery. 2005; 56:130–137. discussion 138. PMID: 15617595.

17. Roundy N, Delashaw JB, Cetas JS. Preoperative identification of the facial nerve in patients with large cerebellopontine angle tumors using high-density diffusion tensor imaging. J Neurosurg. 2012; 116:697–702. PMID: 22283188.

18. Samii M, Koerbel A, Safavi-Abbasi S, Di Rocco F, Samii A, Gharabaghi A. Using an end-to-side interposed sural nerve graft for facial nerve reinforcement after vestibular schwannoma resection. Technical note. J Neurosurg. 2006; 105:920–923. PMID: 17405267.

19. Sluyter S, Graamans K, Tulleken CA, Van Veelen CW. Analysis of the results obtained in 120 patients with large acoustic neuromas surgically treated via the translabyrinthine-transtentorial approach. J Neurosurg. 2001; 94:61–66. PMID: 11147899.

20. Taoka T, Hirabayashi H, Nakagawa H, Sakamoto M, Myochin K, Hirohashi S, et al. Displacement of the facial nerve course by vestibular schwannoma : preoperative visualization using diffusion tensor tractography. J Magn Reson Imaging. 2006; 24:1005–1010. PMID: 17031835.

21. Wu H, Sterkers J. Translabyrinthine removal of large acoustic neuromas in young adults. Auris Nasus Larynx. 2000; 27:201–205. PMID: 10808105.

Fig. 1
A : The preoperative axial enhanced T1-weighted magnetic resonance image shows a vestibular schwannoma on the right cerebellopontine angle (arrow). B : The preoperative diffusion tensor imaging of facial nerve tractography shows that the facial nerve was displaced anterior to the tumor mass (red color).

Fig. 2
A : Intraoperative finding shows facial nerve was transected. B : There was a first attempt to connect the injured facial nerve through a primary end-to-end anastomosis by suture, but it failed due to the thin and fragile facial nerve (arrow). C : Two pieces of Tachocomb2® were placed to surround the injured facial nerve like sandwich (arrow). And then, fibrin glue was used (arrow) (D).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download