Abstract
Meningioma is a common primary tumor of central nervous system. However, extracranial extension of the intracranial meningioma is unusual, and mostly accompanied the osteolytic change of the skull. We herein describe an atypical meningioma having extracranial extension with hyperostotic change of the skull. The patient was a 72-year-old woman who presented a large mass in the right frontal scalp and left hemiparesis. Brain magnetic resonance imaging and computed tomography scans revealed an intracranial mass, diffuse meningeal thickening, hyperostotic change of the skull with focal extension into the right frontal scalp. She underwent total removal of extracranial tumor, bifrontal craniectomy, and partial removal of intracranial tumor followed by cranioplasty. Tumor pathology was confirmed as atypical meningioma, and she received adjuvant radiotherapy. In this report, we present and discuss a meningioma en plaque of atypical histopathology having an extracranial extension with diffuse intracranial growth and hyperostotic change of the skull.
Meningiomas are the second common central nervous system (CNS) neoplasm in adults and account for 15-20% of all primary brain tumors. Although most meningiomas are benign, approximately 10% demonstrates a more aggressive clinical behavior and are classified as non-benign meningiomas8). Extracranial meningiomas are 1-2% of all meningioma, and the majority has a secondary extension of the primary intracranial tumors and accompanies the osteolytic change of the skull7,10). Some intracranial meningiomas may extend to skull leading to cranial hyperostosis. Osteoblastic intraosseous meningiomas may induce hyperostosis3). Meningioma en plaque (MEP) represents an infiltration to the dura and sometimes invades the bone with the intraosseous tumor growth leading to significant hyperostosis. But, the concomitant appearance of scalp meningioma associated with intracranial atypical MEP accompanying hyperostosis has been rarely described. We herein describe an uncommon atypical MEP, which accompanied intracranial growth and extracranial extension with hyperostotic change of the skull.
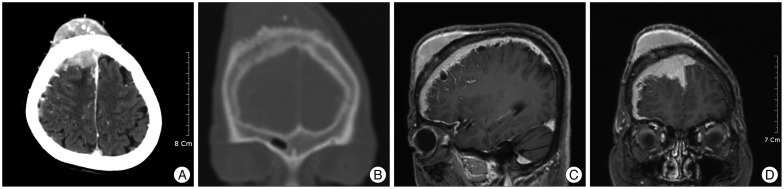
A 72-year-old woman presented with a large mass in the right frontal scalp and left sided-motor weakness. The mass was progressively enlarged over 4 years, and motor weakness has been developed since 6 months before admission. Scalp tumor was a well-defined, subcutaneous firm mass, and located in the right frontal area adjacent to the midline. The enhanced computed tomography (CT) scans showed the well-enhanced mass, sized 6.2×2.6×5.1 cm in the right frontal scalp, and diffuse growth of intracranial tumor accompanying the hyperostotic change of the skull bone (Fig. 1A, B). Brain magnetic resonance images revealed the well-enhanced tumors with bony infiltration in the right frontal region, diffuse meningeal thickening with multiple cystic changes in the right hemisphere (Fig. 1C, D). Main feeder of tumor was the right external carotid artery, and anterior part of the superior sagittal sinus was compressed by tumor. She underwent a total removal of the scalp tumor and bifrontal craniectomy under impression of extracranial extension of intracranial meningioma. In consideration of the patient's age and tumor location adjacent to the eloquent area, intracranial tumor with thickened dura mater was partially removed, and followed by duroplasty with cranioplasty. The outer table of skull was rough, and tumor was firm and tan-gray color intraoperatively. The tumor was diffusely adhere to the brain surface was observed and it was not possible to undergo total resection without cortical injury.
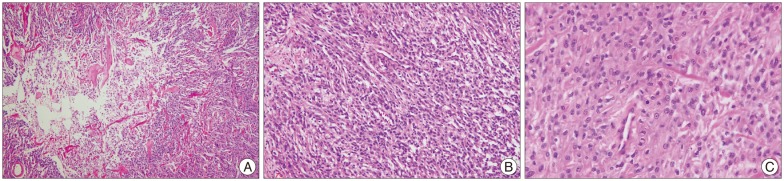
Histopathological examination of the tumor specimen demonstrated a mass composed of geographic necrosis, increased cellularity, patternless or sheet like pattern and invasion, mild to moderate pleomorphism. Four or more mitotic cells per 10 high-power fields were seen, but mitotic numbers were not over twenty (Fig. 2). Immunohistochemical staining was positive for vimentin and epithelial membrane antigen, and negative for cytokeratins and S-100 protein. Conclusively, these findings were compatible with an atypical meningioma. Postoperative adjuvant radiotherapy was performed immediately and she received a total dose of 44.8 Gy in 28 fractions for 6 weeks. After adjuvant radiation therapy, she complained of aggravation of motor weakness in the left arm. But, her motor weakness was transient and she was uneventful at discharge.
Meningiomas are the most common non-glial intracranial primary tumors. MEPs are presumed to account for 2-4% of intracranial meningiomas4). These tumors are defined by an intraosseus tumor growth leading to significant hyperostosis and a widespread, carpet-like, soft-tissue growth at the dura. MEP is predominantly found in the middle age, with the peak incidence between fourth and fifth decade years. The female-to-male ratio of incidence is about 3-5 : 117).
MEP is a specific clinicopathological entity, which although locally invasive, usually bears the histology of World Health Organization (WHO) grade I meningioma1). Atypical (WHO grade II) meningiomas constitute approximately 5-7% of meningiomas6). Especially, atypical meningioma is rare but existed among MEPs. Li et al.13) reported 2 cases of MEP with atypical pathology out of 37 MEPs in the sphenoid wing. Hyperostosis is a well-known sign of meningiomas, which is observed in 4.5% of all types, but is more frequently observed in meningioma en plaque with an incidence of 13 to 49%5). The histological type of meningiomas appears to have no relationship with hyperostosis, but the various histological types are found as MEP, which are frequently associated with hyperostosis9). In addition, there is no relationship between the hyperostotic pattern and the histological type of meningioma11).
The differential diagnosis includes a number of mesenchymal and epithelial tumors in the head, such as, paraganglioma, carcinoma, melanoma, schwannoma, olfactory neuroblastoma, fibrous dysplasia, osteoma, osteoblastic metastasis, Paget's disease, hyperostosis frontalis interna, erythroid hyperplasia, and sarcoidosis or others depending on the anatomic site of involvement16). Inward bulging of the inner aspect of the hyperostotic bone, irregularity of the bone surface, and intracranial changes are important imaging features that can be used in differential diagnosis of MEP2).
The most successful outcome of treatment for atypical meningioma can be obtained by the early and extensive surgical resection. Surgical intervention consists of the removal of all involved lesions including bones, dura, muscles, intracranial and intraorbital components. Radiation therapy has been suggested to yield an improvement in patient survival with some meningiomas of the CNS15). Goyal et al.6) reported that radiation therapy was associated with less recurrence : 22 (78.5%) of the 28 patients who did not develop recurrence were the group that received radiation therapy. Park et al.15) reported that progression-free survival was significantly higher in patients undergoing radiation therapy after surgical resection than those not undergoing radiation therapy (58.7% vs. 44.3%, at 5 years, p=0.029). Maroon et al.14) have recommended postoperative radiation therapy when tumors were subtotally removed. It has been also indicated if there is the dural or cavernous sinus invasion of tumor, or tumor recurrence on neuroimaging. Radiation therapy for atypical meningioma should be considered if there are residual tumors or the recurrent tumor after initial radical removal of bone and tumor. In our case, radiation therapy has been also performed, however, patient outcome was not reached to satisfaction. The prognosis of meningioma is generally favorable if the complete excision is attempted12). However, rare cases of meningioma like atypical MEP are aggressive and total resection is unfeasible. A few of meningiomas can develop as a malignant pathological type.
Meningiomas are moslty benign intracranial lesions and their coexistence with extracranial extension of atypical MEP is rarely reported. We hereby describe and discuss an atypical MEP having an extracranial extension with diffuse intracranial growth. Total resection of tumor is a choice of treatment for meningioma, however, management should be tailored depending on the status of patient.
References
1. Basu K, Majumdar K, Chatterjee U, Ganguli M, Chatterjee S. En plaque meningioma with angioinvasion. Indian J Pathol Microbiol. 2010; 53:319–321. PMID: 20551544.

3. Daffner RH, Yakulis R, Maroon JC. Intraosseous meningioma. Skeletal Radiol. 1998; 27:108–111. PMID: 9526778.

4. De Jesús O, Toledo MM. Surgical management of meningioma en plaque of the sphenoid ridge. Surg Neurol. 2001; 55:265–269. PMID: 11516463.

5. Doyle WF, Rosegay H. Meningioma en plaque with hyperostosis : case report. Mil Med. 1972; 137:196–198. PMID: 4623534.
6. Goyal LK, Suh JH, Mohan DS, Prayson RA, Lee J, Barnett GH. Local control and overall survival in atypical meningioma : a retrospective study. Int J Radiat Oncol Biol Phys. 2000; 46:57–61. PMID: 10656373.

7. Iglesias ME, Vázquez-Doval J, Idoate MA, Vanaclocha V, Idoate F, Quintanilla E. Intracranial osteolytic meningioma affecting the scalp. J Am Acad Dermatol. 1996; 35:641–642. PMID: 8859303.

8. Jo K, Park HJ, Nam DH, Lee JI, Kong DS, Park K, et al. Treatment of atypical meningioma. J Clin Neurosci. 2010; 17:1362–1366. PMID: 20800497.

9. Kashimura H, Beppu T, Wada T, Yoshida Y, Suzuki M, Ogawa A. [A case of meningioma en plaque : review of 73 cases]. No Shinkei Geka. 1997; 25:1097–1100. PMID: 9430144.
10. Kim H, Jung TY, Kim IY, Lee JK. Two cases of primary osteolytic intraosseous meningioma of the skull metastasizing to whole skull and the spine. J Korean Neurosurg Soc. 2012; 51:151–154. PMID: 22639712.

11. Kim KS, Rogers LF, Goldblatt D. CT features of hyperostosing meningioma en plaque. AJR Am J Roentgenol. 1987; 149:1017–1023. PMID: 3118666.

12. Kumar S, Dhingra PL, Gondal R. Ectopic meningioma of the paranasal sinuses. Childs Nerv Syst. 1993; 9:483–484. PMID: 8124679.

13. Li Y, Shi JT, An YZ, Zhang TM, Fu JD, Zhang JL, et al. Sphenoid wing meningioma en plaque : report of 37 cases. Chin Med J (Engl). 2009; 122:2423–2427. PMID: 20079153.
14. Maroon JC, Kennerdell JS, Vidovich DV, Abla A, Sternau L. Recurrent spheno-orbital meningioma. J Neurosurg. 1994; 80:202–208. PMID: 8283257.

15. Park HJ, Kang HC, Kim IH, Park SH, Kim DG, Park CK, et al. The role of adjuvant radiotherapy in atypical meningioma. J Neurooncol. 2013; 115:241–247. PMID: 23949108.

16. Pieper DR, Al-Mefty O. Management of intracranial meningiomas secondarily involving the infratemporal fossa : radiographic characteristics, pattern of tumor invasion, and surgical implications. Neurosurgery. 1999; 45:231–237. discussion 237-238. PMID: 10449066.

17. Schick U, Bleyen J, Bani A, Hassler W. Management of meningiomas en plaque of the sphenoid wing. J Neurosurg. 2006; 104:208–214. PMID: 16509494.

Fig. 1
Preoperative brain enhanced computed tomography (CT) scans and magnetic resonance (MR) image. A : Axial CT scan displaying the well-enhanced mass in the right frontal area extended through the skull diffusely. B : Coronal CT scan showing the frontal bulging with rough outer table by the hyperostotic change. C and D : Sagittal and coronal T2-weighted MR images revealing the ill-defined tumor compressing the right frontal lobe and the marginal multi-cystic mass in the right hemisphere.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download