Abstract
Coarctation and occlusion of the aorta is a rare condition that typically presents with hypertension or cardiac failure. However, neuropathy or myelopathy may be the presenting features of the condition when an intraspinal subarachnoid hemorrhage has compressed the spinal cord causing ischemia. We report two cases of middle-aged males who developed acute non-traumatic paraplegia. Undiagnosed congenital abnormalities, such as aortic coarctation and occlusion, should be considered for patients presenting with nontraumatic paraplegia in the absence of other identifiable causes. Our cases suggest that spinal cord ischemia resulting from acute spinal subarachnoid hemorrhage and can cause paraplegia, and that clinicians must carefully examine patients presenting with nontraumatic paraplegia because misdiagnosis can delay initiation of the appropriate treatment.
Acute aortic coarctation with occlusion of the aorta is rare condition that has an extremely poor prognosis and high incidence of neurovascular complications. Paraplegia may be caused by intraspinal subarachnoid hemorrhage and spinal cord ischemia caused by aortic thrombosis and occlusion1,10,13). We present two cases admitted to our Emergency Department with flaccid paraplegia that were diagnosed with acute aortic coarctation and occlusion.
A 45-year-old male was admitted for abrupt paraplegia that occurred during exercise in a fitness club. His past medical history included diabetes and hypertension. On the initial physical examination, the Emergency Department physician detected complete flaccid paraplegia. The patient had loss of pain and temperature sensation and absence of tendon reflexes below the 156T4 level. Routine laboratory tests showed no abnormal findings. On radiographic examination, a chest X-ray showed cardiomegaly, pulmonary congestion, and mediastinal widening. Magnetic resonance imaging (MRI) (Fig. 1) revealed an acute spinal subarachnoid hematoma extending from C2 to T4 with spinal cord compression and numerous dilated anterior spinal arteries at the C6-T3 level. A computed tomography (CT) angiogram (Fig. 2) showed a tortuous aortic coarctation with an extensive network of collaterals. The patient was hemodynamically stable and showed gradual improvement in the neurological deficit of the upper extremities following anti-edematous therapy including intravenous corticosteroid injections; however, the treatment failed to reverse the neurological condition. Endovascular intervention was recommended, and the patient was referred for surgical repair of the aortic coarctation. However, he refused and was transferred to another tertiary hospital.
A 58-year-old male admitted to the Emergency Department complaining of acute weakness in both low extremities and severe low back pain after jumping to the ground from a height of one meter 30 min before arriving at the hospital. His past medical history was not significant. On the initial physical examination, the Emergency Department physician detected complete flaccid paraplegia. The patient had loss of pain and temperature sensation and the absence of tendon reflexes below the L1 level. Initial routine laboratory tests revealed no abnormal findings.
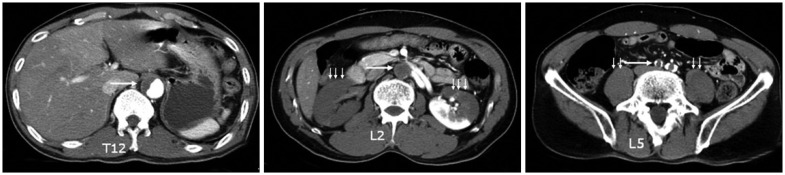
On radiographic examination, the lumbar spine radiographs showed spondylosis with aortic calcification. Lumbar CT revealed spondylolysis on L5 and the lumbar MRI revealed disc degeneration at L4--5 and L5--S1 without spinal cord compression or cord swelling (Fig. 3).
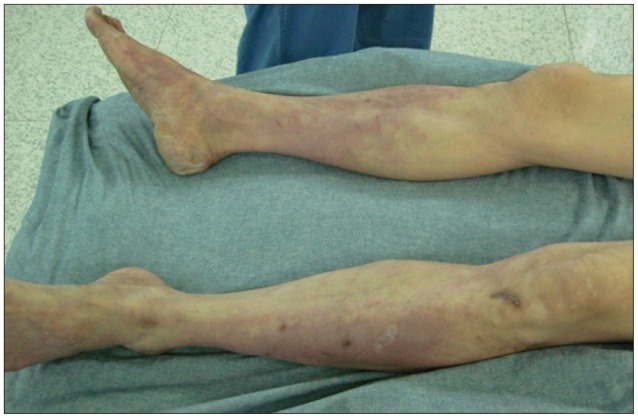
Subsequently, we detected coldness and pallor in the lower extremities (Fig. 4) and the absence of the dorsalis pedis arterial pulse. A CT angiography of the abdominal aorta and lower extremities performed immediately revealed aortoiliac thrombotic occlusion with renal infarction (Fig. 5).
The patient underwent aortobifemoral bypass surgery performed by a vascular surgeon. However, he died 2 days later as a result of multiple organ failure caused by compartment syndrome.
Aortic coarctation accounts for approximately 7% of congenital heart disease20). The development of collateral circulation and a connection between the prestenotic and poststenotic thoracic aorta are signs of coarctation. Furthermore, collateral vessels may form aneurysmal dilatations as a result of deficiencies in muscle and elastic tissue, as occurs in long-standing arterial hypertension, which may cause a rupture4).
Patients with coarctation are prone to neurovascular complications. Intracranial hemorrhage of an aneurysm occurs in approximately 12% of untreated cases, and intracranial subarachnoid hemorrhage accounts for 7% of deaths in patients with aortic coarctation2,5). Spinal hemorrhage as a complication of coarctation is rare compared with intracranial hemorrhage, and few cases have been reported. The clinical presentation includes sudden back or neck pain and neurological deficits as serious as paraplegia or quadriplegia. Most cases involve spinal arachnoid hemorrhage following rupture of an aneurysm and an abnormally dilated spinal artery or collateral vessel2,8,14,22).
MRI is a noninvasive technique with immense diagnostic potential that is a suitable primary imaging method for patients with spontaneous paraparesis. However, MRI images cannot reliably identify the blood/arachnoid interface when they are in close proximity. Moreover, as in cases of ventral hematoma, it is not possible to determine whether the hematoma is located in the epidural, subdural, and subarachnoid space.
Myelopathy associated with aortic coarctation requires individualized treatment. Surgical decompression is often necessary when the patient's neurological status is compromised or in rapid deterioration, whereas conservative treatment is sufficient for patients in good general and neurological condition. About 50% of surgically indicated patients are treated conservatively as a result of poor general conditions5). The patient in our first case had abrupt paraplegia and was in good general health; however, the hematoma was located ventrally, and the patient was reluctant to undergo surgery, thus conservative treatment was commenced. Surgery is the conventional treatment for aortic coarctation; however, recent evidence suggests that percutaneous endovascular stents are associated with a low-residual gradient and a low rate of restenosis immediately and at follow-up, and are a good alternative to surgery in anatomically suitable adolescents and adults7). We chose surgery as the first-line treatment because of the tortuous and stenotic nature of the coarctation.
Acute aortic occlusion is rare in patients with no history of peripheral vascular disease, and the condition is associated with high morbidity and mortality rates. Possible etiologies include in situ thrombosis, aortic saddle embolus, and an intimal flap that causes aortic dissection and thrombosis9,16,19). Aortic dissection and reparative vascular surgery are well-established causes of spinal cord ischemia1,9,10,11,16). Investigations of the relationship between paraplegia and acute aortic thrombosis have suggested that aortic thrombosis may be caused by in situ thrombosis, cancer, chemotherapy ischemic cardiomyopathy13,18,19,21,23). In our case, the CT angiography from the ninth thoracic vertebra to the lower extremities confirmed aortoiliac thrombotic occlusion. However, we cannot exclude the possibility of abdominal thrombosis related to thoracic aortic dissection because the area above T9 was not included in the CT angiogram.
The classic signs and symptoms of acute aortic occlusion are called the "five Ps" : pain, pulselessness, pallor, paresthesias, and paralysis. However, cases of intestinal ischemia6,17) and painless paraplegia1,10) have been reported. Aggressive medical treatment must be administered to patients who show evidence of intestinal ischemia on radiographic evaluation. The initial symptoms are generally paralysis of the lower extremities caused by a lesion that compresses the spinal cord ventrally, and an incorrect diagnosis may delay definite treatment21). Absence of the femoral or more distal arterial pulse can be an important differential diagnostic indicator. In our case, the patient complained of lower extremity weakness and was initially referred to an emergency medicine physician. The admitting physician was unsure of the origin of the symptoms and initially consulted several departments including orthopedic surgery and neurology. However, despite several initial tests, including lumbar CT and MRI, the correct diagnosis was not made until the physician observed coldness and pallor in the lower extremities and the absence of the dorsalis pedis arterial pulse. In our view, a rare disease entity, such as our cases, reveals a weakness in departmentalized clinical services in the hospital. It is likely that a number of clinical departments have treated cases aortic thrombotic occlusion. Upon re-examination, we suspected that the symptoms were the result of spinal cord ischemia, such as anterior spinal artery syndrome. Spinal cord ischemia is characterized by segmental necrosis of the gray and adjacent white matter, whereas the peripheral white matter is spared. Axonal swelling, myelin degeneration, petechial hemorrhage, and macrophage infiltration are histological indicators of spinal cord ischemia12). Furthermore, the CT angiography revealed renal infarction, which has a poor prognosis. It is important to note that pallor, pulselessness, and coldness are the primary symptoms that distinguish vascular lesions from other cardinal diseases that cause pain or weakness in the lower extremities.
It is generally accepted that the earlier the revascularization, the better the result. However, there may be an 8-h timeframe before the results of revascularization are poor3). Furthermore, revascularization may be followed by acute systemic reactions. Breakdown products of rhabdomyolysis such as myoglobin, lactic acid dehydrogenase, creatine phosphokinase, and excessive potassium and newly formed clots in the venous system of a previously ischemic limb are released into the circulation and may damage the lungs, myocardium, liver, and kidneys. Moreover, compartment syndrome is common after revascularization of a limb15). We believe a minor impact, such as jumping to the ground, may have induced a thoracic embolus in our second patient, and the massive aortic occlusion caused simultaneous rupture of the artery of Adamkiewicz, the anterior spinal artery, and segmental spinal arteries resulting in acute hypoperfusion of the spinal cord and ischemia. However, we cannot rule out the possibility of the original occlusion below the artery of Adamkiewicz or a thoracic aortic dissection. The patient did not feel pain in the lower extremities as a result of the subsequent paraplegia, and because the condition is rare, the clinician, who was an emergency room specialist, overlooked the possibility of aortic occlusion. It is critically important that clinicians recognize the possibility of a vascular event in such cases because delayed diagnosis may contribute to a poorer prognosis.
We report here two rare cases of acute paraplegia resulting from acute coarctation and occlusion of the aorta. Coarctation and occlusion of the aorta must be considered for patients complaining of acute lower extremity weakness with or without pain because early diagnosis and appropriate treatment are essential for a favorable outcome. From this point of view, the clinician must carefully examine the patient, palpate the affected area, and consider the possibility of vascular origin. In our experience, coldness of the lower extremities the most important early sign of aortic thrombotic occlusion with the exception of the five Ps : pain, pulselessness, pallor, paresthesias, and paralysis.
References
1. Aktas C, Cinar O, Ay D, Gürses B, Hasmanoglu H. Acute aortic dissection with painless paraplegia : report of 2 cases. Am J Emerg Med. 2008; 26:631.e3–631.e5. PMID: 18534306.
2. Banna MM, Rose PG, Pearce GW. Coarctation of the aorta as a cause of spinal subarachnoid hemorrhage. Case report. J Neurosurg. 1973; 39:761–763. PMID: 4759663.
3. Blaisdell FW, Steele M, Allen RE. Management of acute lower extremity arterial ischemia due to embolism and thrombosis. Surgery. 1978; 84:822–834. PMID: 715701.
4. Campbell M. Natural history of coarctation of the aorta. Br Heart J. 1970; 32:633–640. PMID: 5470045.

5. Domenicucci M, Ramieri A, Paolini S, Russo N, Occhiogrosso G, Di Biasi C, et al. Spinal subarachnoid hematomas : our experience and literature review. Acta Neurochir (Wein). 2005; 147:741–750. discussion 750. PMID: 15711890.
6. Edwards KC, Katzen BT. Superior mesenteric artery syndrome due to large dissecting abdominal aortic aneurysm. Am J Gastroenterol. 1984; 79:72–74. PMID: 6691326.
7. Godart F. Intravascular stenting for the treatment of coarctation of the aorta in adolescent and adult patients. Arch Cardiovasc Dis. 2011; 104:627–635. PMID: 22152515.

8. Hino H, Maruyama H, Inomata H. A case of spinal artery aneurysm presenting transverse myelopathy associated with coarctation of the aorta. Rinsho Shinkeigaku. 1989; 29:1009–1012. PMID: 2689031.
9. Hölper P, Hyhlik-Dürr A, Kotelis D, von Tengg-Kobligk H, Böckler D. Paraplegia after spontaneous dissection of the abdominal aorta. Vasa. 2009; 38:254–258. PMID: 19736638.

10. Inamasu J, Hori S, Yokoyama M, Funabiki T, Aoki K, Aikawa N. Paraplegia caused by painless acute aortic dissection. Spinal Cord. 2000; 38:702–704. PMID: 11114779.

11. Karacostas D, Anthomelides G, Ioannides P, Psaroulis K, Psaroulis D. Acute paraplegia in painless aortic dissection. Rich imaging with poor outcome. Spinal Cord. 2010; 48:87–89. PMID: 19528998.

12. Kelley CE. Nontraumatic abdominal aortic thrombosis presenting with anterior spinal artery syndrome and pulmonary edema. J Emerg Med. 1991; 9:233–237. PMID: 1861056.

13. Kochar G, Kotler MN, Hartman J, Goldberg SE, Parry W, Parameswaran R, et al. Thrombosed aorta resulting in spinal cord ischemia and paraplegia in ischemic cardiomyopathy. Am Heart J. 1987; 113:1510–1513. PMID: 3591620.

14. Lins E, Vlajic I, Wappenschmidt J. Spinal subarachnoid hemorrhage in a case of stenosis of the aorta (author's transl). Rontgenblatter. 1977; 30:239–244. PMID: 866925.
15. Lusby RJ, Wylie EJ. Acute lower limb ischemia : pathogenesis and management. World J Surg. 1983; 7:340–386. PMID: 6880224.
16. Nandeesh BN, Mahadevan A, Santosh V, Yasha TC, Shankar SK. Acute aortic dissection presenting as painful paraplegia. Clin Neurol Neurosurg. 2007; 109:531–534. PMID: 17475398.

17. O'Dell KB, Hakim SN. Dissecting thoracic aortic aneurysm in a 22-year-old man. Ann Emerg Med. 1990; 19:316–318. PMID: 2310071.
18. Poirée S, Monnier-Cholley L, Tubiana JM, Arrivé L. Acute abdominal aortic thrombosis in cancer patients. Abdom Imaging. 2004; 29:511–513. PMID: 15024514.

19. Surowiec SM, Isiklar H, Sreeram S, Weiss VJ, Lumsden AB. Acute occlusion of the abdominal aorta. Am J Surg. 1998; 176:193–197. PMID: 9737631.

20. Swan L, Wilson N, Houston AB, Doig W, Pollock JC, Hillis WS. The long-term management of the patient with an aortic coarctation repair. Eur Heart J. 1998; 19:382–386. PMID: 9568441.

21. Triantafyllopoulos GK, Athanassacopoulos M, Maltezos C, Pneumaticos SG. Acute infrarenal aortic thrombosis presenting with flaccid paraplegia. Spine (Phila Pa 1976). 2011; 36:e1042–e1045. PMID: 21289558.

22. Watson AB. Spinal subarachnoid haemorrhage in patient with coarctation of aorta. Br Med J. 1967; 4:278–279. PMID: 6054005.

Fig. 1
Magnetic resonance imaging reveals a large acute spinal subarachnoid hematoma extending from C2 to T4 with spinal cord compression and numerous dilated anterior spinal arteries at the C6--T3 levels (white asterisk).
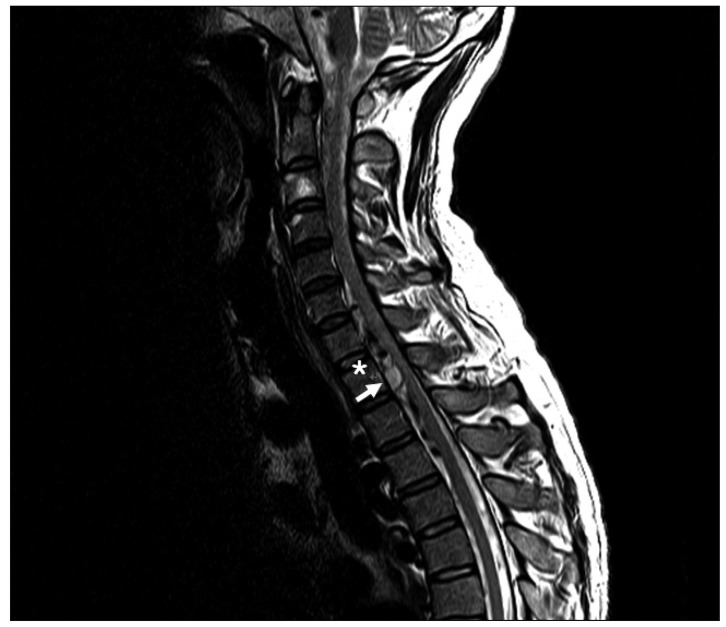
Fig. 2
The computed tomography angiogram reveals a tortuous aortic coarctation (white arrow) with an extensive network of collaterals (arrowheads).
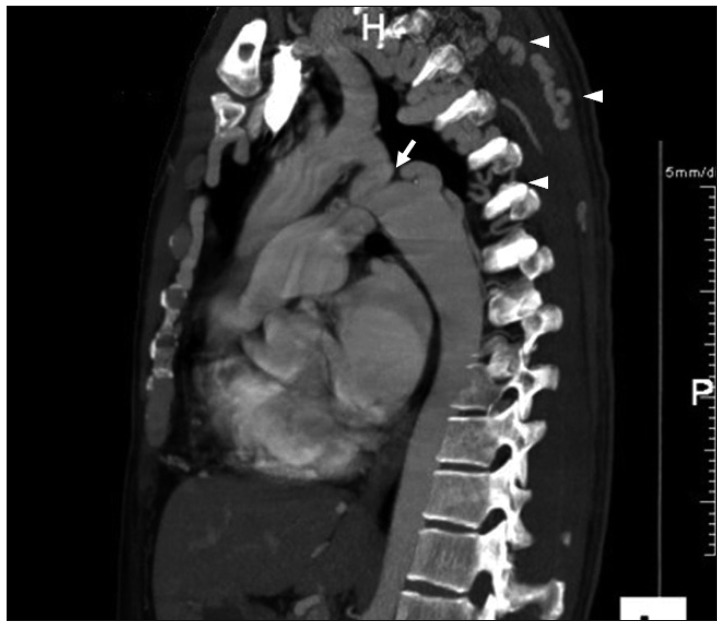
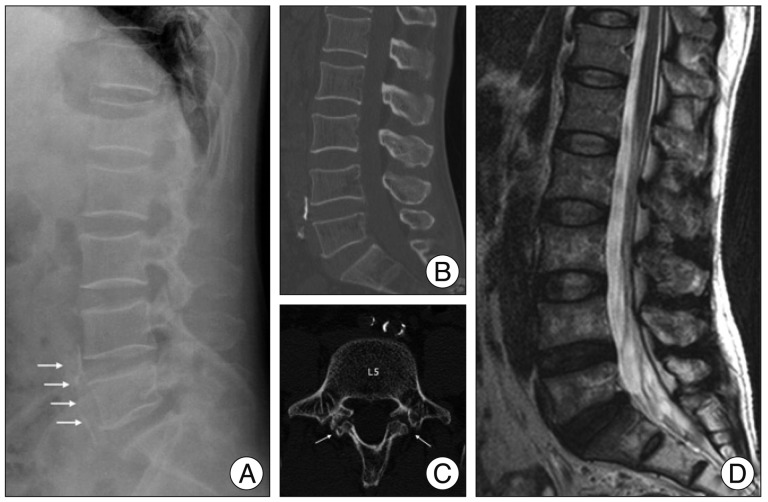
Fig. 3
Preoperative radiographs. Lateral lumbar spine radiograph (A) shows some spondylosis with aortic calcification (arrows). Lumbar CT (B and C) shows spondylolysis on L5 (arrows). T2-weighted MRI (D) reveals disc degeneration of L4--5 and L5--S1 without compressive lesion of the spinal cord or cord swelling.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download