1. Balentine JD. Pathology of experimental spinal cord trauma. I. The necrotic lesion as a function of vascular injury. Lab Invest. 1978; 39:236–253. PMID:
713489.
2. Balentine JD. Pathology of experimental spinal cord trauma. II. Ultrastructure of axons and myelin. Lab Invest. 1978; 39:254–266. PMID:
713490.
3. Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996; 139:244–256. PMID:
8654527.

4. Brederlau A, Correia AS, Anisimov SV, Elmi M, Paul G, Roybon L, et al. Transplantation of human embryonic stem cell-derived cells to a rat model of Parkinson's disease : effect of in vitro differentiation on graft survival and teratoma formation. Stem Cells. 2006; 24:1433–1440. PMID:
16556709.

5. Brüstle O, Jones KN, Learish RD, Karram K, Choudhary K, Wiestler OD, et al. Embryonic stem cell-derived glial precursors : a source of myelinating transplants. Science. 1999; 285:754–756. PMID:
10427001.

6. Bug G, Gül H, Schwarz K, Pfeifer H, Kampfmann M, Zheng X, et al. Valproic acid stimulates proliferation and self-renewal of hematopoietic stem cells. Cancer Res. 2005; 65:2537–2541. PMID:
15805245.

7. Chen PS, Peng GS, Li G, Yang S, Wu X, Wang CC, et al. Valproate protects dopaminergic neurons in midbrain neuron/glia cultures by stimulating the release of neurotrophic factors from astrocytes. Mol Psychiatry. 2006; 11:1116–1125. PMID:
16969367.

8. Chuang DM, Leng Y, Marinova Z, Kim HJ, Chiu CT. Multiple roles of HDAC inhibition in neurodegenerative conditions. Trends Neurosci. 2009; 32:591–601. PMID:
19775759.

9. Dash PK, Orsi SA, Zhang M, Grill RJ, Pati S, Zhao J, et al. Valproate administered after traumatic brain injury provides neuroprotection and improves cognitive function in rats. PLoS One. 2010; 5:e11383. PMID:
20614021.

10. Feng HL, Leng Y, Ma CH, Zhang J, Ren M, Chuang DM. Combined lithium and valproate treatment delays disease onset, reduces neurological deficits and prolongs survival in an amyotrophic lateral sclerosis mouse model. Neuroscience. 2008; 155:567–572. PMID:
18640245.

11. Gage FH, Coates PW, Palmer TD, Kuhn HG, Fisher LJ, Suhonen JO, et al. Survival and differentiation of adult neuronal progenitor cells transplanted to the adult brain. Proc Natl Acad Sci U S A. 1995; 92:11879–11883. PMID:
8524867.

12. Go HS, Kim KC, Choi CS, Jeon SJ, Kwon KJ, Han SH, et al. Prenatal exposure to valproic acid increases the neural progenitor cell pool and induces macrocephaly in rat brain via a mechanism involving the GSK-3β/β-catenin pathway. Neuropharmacology. 2012; 63:1028–1041. PMID:
22841957.

13. Gorio A, Gokmen N, Erbayraktar S, Yilmaz O, Madaschi L, Cichetti C, et al. Recombinant human erythropoietin counteracts secondary injury and markedly enhances neurological recovery from experimental spinal cord trauma. Proc Natl Acad Sci U S A. 2002; 99:9450–9455. PMID:
12082184.

14. Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, et al. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci U S A. 2004; 101:18117–18122. PMID:
15608062.

15. Inoue T, Kawaguchi S, Kurisu K. Spontaneous regeneration of the pyramidal tract after transection in young rats. Neurosci Lett. 1998; 247:151–154. PMID:
9655615.

16. Iseda T, Nishio T, Kawaguchi S, Yamanoto M, Kawasaki T, Wakisaka S. Spontaneous regeneration of the corticospinal tract after transection in young rats : a key role of reactive astrocytes in making favorable and unfavorable conditions for regeneration. Neuroscience. 2004; 126:365–374. PMID:
15207354.

17. Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisén J. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999; 96:25–34. PMID:
9989494.

18. Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K, et al. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. 2005; 25:4694–4705. PMID:
15888645.

19. Kikukawa S, Kawaguchi S, Mizoguchi A, Ide C, Koshinaga M. Regeneration of dorsal column axons after spinal cord injury in young rats. Neurosci Lett. 1998; 249:135–138. PMID:
9682835.

20. Kim HJ, Rowe M, Ren M, Hong JS, Chen PS, Chuang DM. Histone deacetylase inhibitors exhibit anti-inflammatory and neuroprotective effects in a rat permanent ischemic model of stroke : multiple mechanisms of action. J Pharmacol Exp Ther. 2007; 321:892–901. PMID:
17371805.

21. Lee H, Shamy GA, Elkabetz Y, Schofield CM, Harrsion NL, Panagiotakos G, et al. Directed differentiation and transplantation of human embryonic stem cell-derived motoneurons. Stem Cells. 2007; 25:1931–1939. PMID:
17478583.

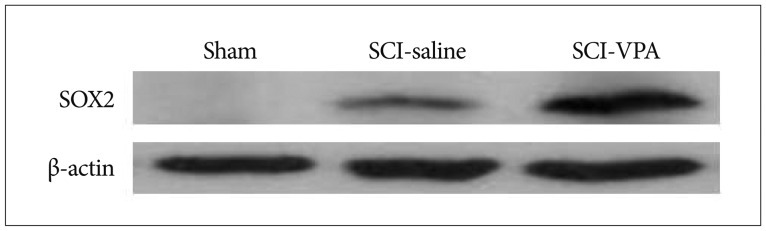
22. Lee HJ, Wu J, Chung J, Wrathall JR. SOX2 expression is upregulated in adult spinal cord after contusion injury in both oligodendrocyte lineage and ependymal cells. J Neurosci Res. 2013; 91:196–210. PMID:
23169458.

23. Lee SM, Yune TY, Kim SJ, Park DW, Lee YK, Kim YC, et al. Minocycline reduces cell death and improves functional recovery after traumatic spinal cord injury in the rat. J Neurotrauma. 2003; 20:1017–1027. PMID:
14588118.

24. Li S, Strittmatter SM. Delayed systemic Nogo-66 receptor antagonist promotes recovery from spinal cord injury. J Neurosci. 2003; 23:4219–4227. PMID:
12764110.

25. Maurer MH, Brömme JO, Feldmann RE Jr, Järve A, Sabouri F, Bürgers HF, et al. Glycogen synthase kinase 3beta (GSK3beta) regulates differentiation and proliferation in neural stem cells from the rat subventricular zone. J Proteome Res. 2007; 6:1198–1208. PMID:
17330951.

26. Nistor GI, Totoiu MO, Haque N, Carpenter MK, Keirstead HS. Human embryonic stem cells differentiate into oligodendrocytes in high purity and myelinate after spinal cord transplantation. Glia. 2005; 49:385–396. PMID:
15538751.

27. Qing H, He G, Ly PT, Fox CJ, Staufenbiel M, Cai F, et al. Valproic acid inhibits Abeta production, neuritic plaque formation, and behavioral deficits in Alzheimer's disease mouse models. J Exp Med. 2008; 205:2781–2789. PMID:
18955571.

28. Ren M, Leng Y, Jeong M, Leeds PR, Chuang DM. Valproic acid reduces brain damage induced by transient focal cerebral ischemia in rats : potential roles of histone deacetylase inhibition and heat shock protein induction. J Neurochem. 2004; 89:1358–1367. PMID:
15189338.

29. Rowland JW, Hawryluk GW, Kwon B, Fehlings MG. Current status of acute spinal cord injury pathophysiology and emerging therapies : promise on the horizon. Neurosurg Focus. 2008; 25:E2. PMID:
18980476.
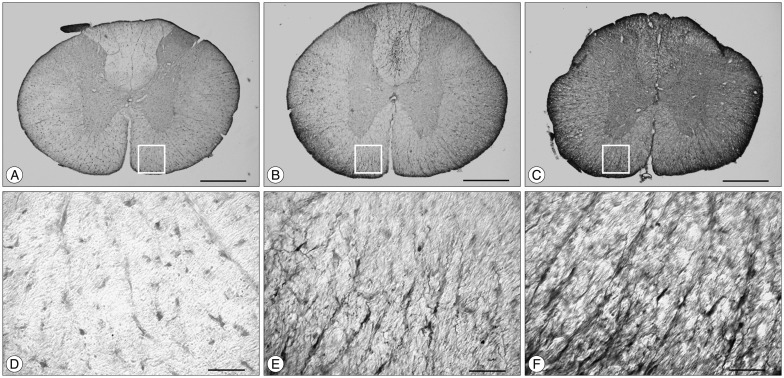
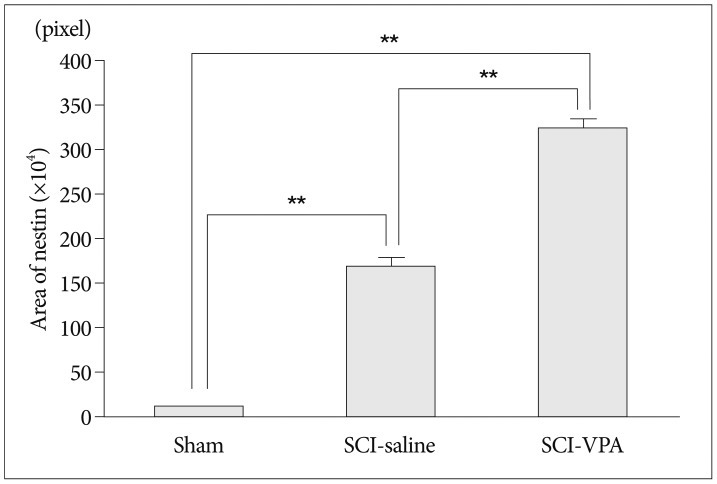
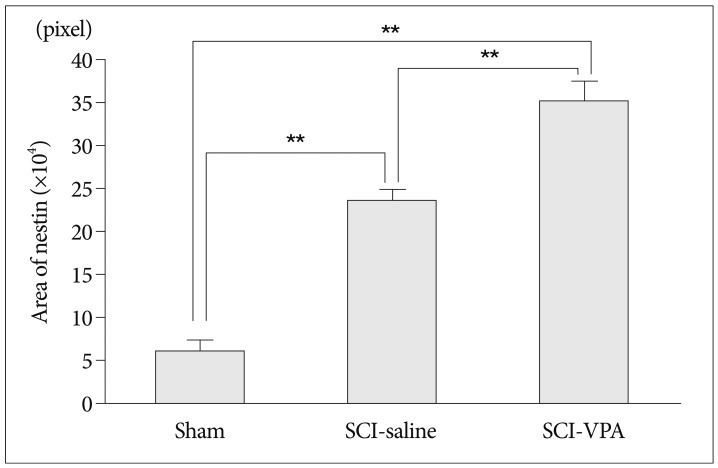
30. Shibuya S, Miyamoto O, Auer RN, Itano T, Mori S, Norimatsu H. Embryonic intermediate filament, nestin, expression following traumatic spinal cord injury in adult rats. Neuroscience. 2002; 114:905–916. PMID:
12379246.

31. Sinn DI, Kim SJ, Chu K, Jung KH, Lee ST, Song EC, et al. Valproic acid-mediated neuroprotection in intracerebral hemorrhage via histone deacetylase inhibition and transcriptional activation. Neurobiol Dis. 2007; 26:464–472. PMID:
17398106.

32. Sugai F, Yamamoto Y, Miyaguchi K, Zhou Z, Sumi H, Hamasaki T, et al. Benefit of valproic acid in suppressing disease progression of ALS model mice. Eur J Neurosci. 2004; 20:3179–3183. PMID:
15579172.

33. Sumner CJ, Huynh TN, Markowitz JA, Perhac JS, Hill B, Coovert DD, et al. Valproic acid increases SMN levels in spinal muscular atrophy patient cells. Ann Neurol. 2003; 54:647–654. PMID:
14595654.

34. Wang Z, Leng Y, Tsai LK, Leeds P, Chuang DM. Valproic acid attenuates blood-brain barrier disruption in a rat model of transient focal cerebral ischemia : the roles of HDAC and MMP-9 inhibition. J Cereb Blood Flow Metab. 2011; 31:52–57. PMID:
20978517.

35. Yu SH, Cho DC, Kim KT, Nam KH, Cho HJ, Sung JK. The neuroprotective effect of treatment of valproic Acid in acute spinal cord injury. J Korean Neurosurg Soc. 2012; 51:191–198. PMID:
22737297.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download