Abstract
Objective
Aneurysms arising from the proximal segment of the anterior cerebral artery (A1) are rare and challenging to treat. The aim of this study was to report our experience with endovascular treatment of A1 Aneurysms.
Methods
From August 2007 through May 2012, eleven A1 aneurysms in eleven patients were treated endovascularly. Six aneurysms were unruptured and 5 were ruptured. One patient with an unruptured A1 aneurysm presented with subarachnoid hemorrhage due to rupture of an anterior communicating artery aneurysm. Procedural data, clinical and angiographic results were reviewed retrospectively.
Results
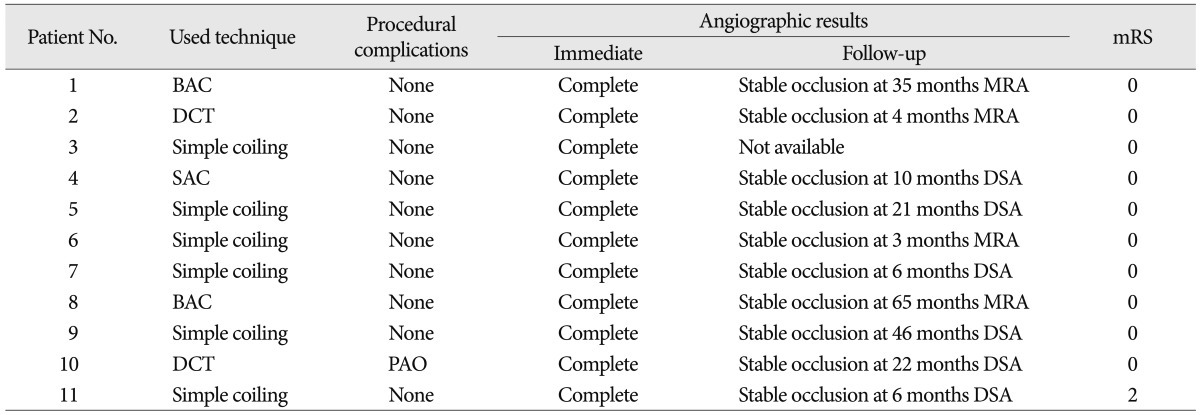
All of the aneurysms were successfully treated with coil embolization. Six were treated with a simple technique while the remaining 5 required adjunctive technique : double catheters (n=2), balloon-assisted (n=2), and stent-assisted (n=1). The immediate angiographic control showed a complete occlusion in all cases. Procedure-related complication occurred in only one patient : parent artery occlusion, which was not clinically significant. All patients had excellent clinical outcomes but one patient was discharged with a slight disability. No neurologic deterioration or bleeding was seen during the follow-up period in this cohort of patients. Follow-up angiography (mean, 20 months) was available in ten patients and revealed stable occlusion in all cases.
A1 aneurysms are located in the proximal segment of the anterior cerebral artery (A1), between the bifurcation of the internal carotid artery (ICA) and the anterior communicating artery. These aneurysms represent less than 1% of all intracranial aneurysms but they are challenging to treat because of their small size and close relationship with perforators2,15,17). With advances in endovascular techniques, coil embolization of ruptured and unruptured intracranial aneurysms has evolved rapidly and has become an efficient treatment technique comparable to surgical clipping. However, very few reports about the endovascular treatment of A1 aneurysms have been published, and the effectiveness and safety of this treatment has not been well-defined. In this study, we present 11 cases which were treated endovascularly for A1 aneurysms and describe clinical and radiologic outcome.
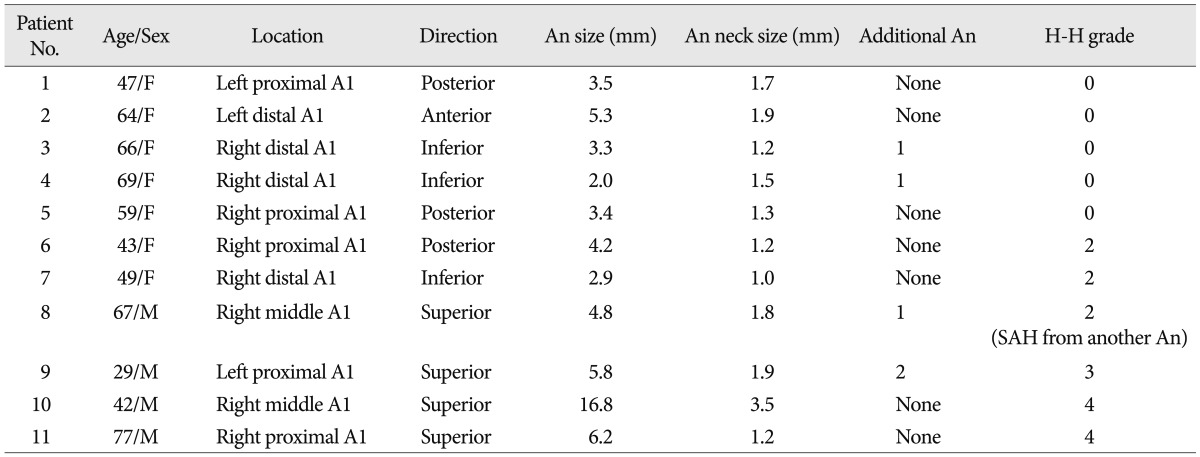
Between August 2007 and May 2012, we retrospectively analyzed the angiographic and clinical records of all patients who underwent endovascular treatment for A1 aneurysms at our institution. Patient demographic data and characteristics of the aneurysms are provided in Table 1. Eleven A1 aneurysms in eleven patients were treated endovascularly. There were four males and seven females with mean age of 55.6 years (range, 29 to 77 years). Dissecting or fusiform aneurysms, aneurysms associated with brain arteriovenous malformations and mycotic aneurysms were excluded from this study. Six aneurysms (54.5%) were unruptured and 5 (45.5%) were ruptured. Six patients (54.5%, 6/11) presented with acute subarachnoid hemorrhage (SAH) from an A1 aneurysm (n=5) or another aneurysm (n=1) : One patient with an unruptured A1 aneurysm presented with SAH due to rupture of an anterior communicating artery aneurysm (Patient 8).
Four patients (36.4%) had multiple aneurysms : One patient had 3 aneurysms and the others had 2. Of these multiple aneurysms (n=5), 2 aneurysms were treated endovascularly at the same time during coil embolization of adjacent unruptured A1 aneurysms (Patient 4, 8).
Regarding the aneurysmal direction, 4 aneurysms projected superiorly (36.4%), 3 projected posteriorly (27.3%), 3 projected inferiorly (27.3%), and 1 projected anteriorly (9%). With regards to aneurysmal location, 5 aneurysms were located in the proximal A1 segment (45.5%), 4 were located in the distal A1 segment (36.4%), and 2 was located in the middle A1 segment (18.1%). Eight aneurysms were located on the right and 3 on the left side. Aneurysm and neck size measured by conventional angiography ranged from 2.0 to 16.8 mm (mean 5.3) and from 1.0 to 3.5 mm (mean 1.7), respectively. There was one large thrombosed aneurysm (Patient 10).
Six patients with SAH were clinically assessed at admission using the Hunt and Hess grade; three patients (50%) were grade II, two (33.3%) were grade IV, and one (16.7%) was grade III.
Indications for endovascular treatment were assessed for every patient by both an interventional neuroradiologist and a vascular neurosurgeon. Informed consent for all interventional procedures was obtained from either patients or their guardians. Five patients without SAH received dual antiplatelet medication including 75 mg of clopidogrel daily and 325 mg of acetylsalicylic acid (ASA) daily over 3 days or a loading dose of 300 mg of clopidogrel and 325 mg of ASA at least 12 hours before the procedure. For the patients presenting with SAH, dual antiplatelet premedication was not prescribed because of the risk of rebleeding.
Systemic heparin was administered in the following manner : a bolus of 3000 IU of heparin was administered intravenously at the beginning of the procedure in cases of unruptured aneurysms or after microcatheter placement into the aneurysms in cases of ruptured aneurysm. An additional 1000 IU bolus of heparin was administered every hour to maintain an activated clotting time (ACT) of longer than 250 seconds throughout the procedure. Every coaxial catheter-flushing fluid was mixed with heparin at a concentration of 1000 IU of heparin per 1000 ml of saline. In all patients, after the procedure, dual antiplatelet therapy of clopidogrel and ASA was also maintained for at least 3 months.
All therapeutic procedures were performed through the right femoral artery under general anesthesia. Electrocardiogram, arterial oxygen saturation, and blood pressure were appropriately monitored. Digital subtraction angiography (DSA) on a biplane neuroangiography unit (Axiom Artis; Siemens AG, Munich, Germany) was performed to evaluate the geometry of the aneurysms. The size of the aneurysm and parent artery was automatically and manually measured. After diagnostic angiography, baseline ACTs were obtained prior to the procedure. A 6 Fr guiding catheter (Envoy; Cordis Neurovascular, Miami, FL, USA) was positioned in the mid-cervical or petrous ICA, and pre-procedural angiograms were obtained in orthogonal planes.
A simple technique, using a single microcatheter, was chosen, and tailored microcatheter shaping was necessary in most cases. A "S-shaped" or "Z-shaped" microcatheter tip was helpful in achieving effective and stable tip positioning in the sac, and inserting coils without early kick-back of the microcatheter. If, however, the simple technique could not be used, adjunctive techniques (double catheters technique or balloon-assisted or stent-assisted) were used to achieve a satisfactory anatomical outcome. Balloon catheters used were the single lumen type (HyperForm; ev3 Neurovascular, Irvine, CA, USA). In one case, a self-expanding Neuroform stent (Boston Scientific/Target, Fremont, CA, USA) covered a neck of A1 aneurysm at the same time during stent-assisted coil embolization of an adjacent anterior communicating aneurysm (Patient 4). Coils of appropriate sizes and shapes were selected according to the target aneurysm. We inserted the coils within the aneurysm as densely as possible, or until another coil could no longer be inserted, without compromising the parent artery lumen, even after angiographic complete obliteration was achieved. After the procedure, multiple angiographic projections were obtained to assess the result. If there were no complications on final angiography performed after 10 minutes, we completed the whole procedure.
Immediately after the procedure, a complete neurological examination was performed with all patients by a vascular neurosurgeon, and all patients underwent non-enhanced brain computed tomographic (CT) scan for evaluation of possible hemorrhagic complications.
Immediate and follow-up angiographic results were analyzed. The rate of occlusion was classified as complete if there was no contrast filling of the sac and neck of the aneurysm, as near-complete if there was slower contrast filling in the neck of the aneurysm, as partial if there was any degree of contrast filling within aneurysm sac. Clinical results were assessed upon discharge from hospital or at the last clinical visit using the modified Rankin Scale (mRS) as follows : 0, no symptoms at all; 1, no significant disability; 2, slight disability; 3, moderate disability; 4, moderately severe disability; 5, severe disability; and 6, dead. Complications were defined as all adverse events related to the procedure and were studied retrospectively using medical and operative reports.
Procedural results and complication of the treatment are shown in Table 2. All of the aneurysms were successfully treated with coil embolization. Six aneurysms were treated with a simple technique. The five remaining aneurysms required adjunctive technique : double catheters (n=2), balloon-assisted (n=2), stent-assisted (n=1).
No procedure-related distal thromboembolism, aneurysm rupture, or death occurred in any patient. Procedure-related complication occurred in only one patient; parent artery occlusion, which occurred during treatment of large thrombosed A1 aneurysm using double catheters technique in the tenth patient (Fig. 1). Because we have concerned about occlusion of the parent artery due to coil extrusion or thrombus migration before procedure, balloon test occlusion was performed to assess the risk of stroke and the patient was tolerable. The post-procedure angiogram confirmed total occlusion of the ipsilateral A1 with no opacification of the sac and collateral circulation distal to occluded A1 provided through the anterior communicating artery. The patient's post-operative period was uneventful.
All patients had excellent clinical outcomes but one patient was discharged with a slight disability. In six patients with SAH, five had no symptoms at discharge (mRS 0). One elderly patient with a ruptured A1 aneurysm was in poor clinical condition (Hunt and Hess grade 4) at admission, nevertheless discharged with mRS 2 disability (Patient 11). Five patients without SAH returned to their previous jobs and remained symptom free. The overall clinical follow-up time ranged from 6 to 65 months (mean 32.7 months). No neurologic deterioration and hemorrhagic complication occurred during the follow-up period in all patients.
The immediate angiographic control showed a complete occlusion in all cases (100%). Follow-up imaging evaluations with conventional angiography or magnetic resonance angiography (MRA), 3 months or longer after endovascular therapy were available for 10 patients. The mean radiological follow-up period was 20 months (range, 3 to 65 months). The overall follow-up angiographic outcomes revealed stable occlusion in all cases. No patient underwent additional treatment during the follow-up period. Representative cases are shown in Fig. 2, 3.
A1 aneurysms are relatively rare, accounting for only about 1% of all cerebral aneurysms2,15,17). Because of their location, small size, and close relationship with perforators, A1 aneurysms were usually treated via craniotomy. There have been several reports on A1 aneurysms treated with craniotomy and overall surgical results were favorable1,2,4,5,8,11,15). In a study of 20 patients with 18 ruptured and 2 unruptured A1 aneurysms, Lee et al.11) reported their surgical outcomes as follows : good recovery in 15 patients, moderate disability in 2 patients, severe disability in 2 patients, and death in 1 patient. Suzuki et al.15) studied the largest series of 38 patients with A1 aneurysms (10 ruptured and 28 unruptured), those who had undergone direct surgery and reported that 28 patients showed excellent operative results, 6 showed good results, 1 showed a fair result, and 3 patients died. On the other hand, they represent a technical challenge for surgical clipping due to the frequent adherence of basal perforating arteries to the aneurysm and thus carry a morbidity of 10-18% depending on the case series15,16).
With advances in endovascular techniques and devices, coil embolization of ruptured and unruptured intracranial aneurysms has evolved rapidly and has become an efficient treatment modality comparable to surgical clipping. However, there are few reports on the endovascular treatment of A1 aneurysms, apparently due to the rarity of these aneurysms3,6,10,12). Lubicz et al.12) treated six A1 aneurysms with endovascular coiling and reported good clinical and anatomical results. They emphasized importance of adjunctive techniques (the use of a balloon or the retrograde approach) to achieve a satisfactory anatomical outcome, because of the location, small size, and close relationship with the perforating arteries of such an aneurysm. More recently, Chang et al. reported the results of 13 cases of A1 aneurysms treated with endovascular treatment with excellent outcomes.
The present study results show that endovascular treatment of A1 aneurysms is feasible and associated with excellent clinical and radiological results, with one procedural complication. Interestingly, this complication, parent artery occlusion, occurred during endosaccular coiling of a large thrombosed A1 aneurysm. Fortunately, because parent artery occlusion was expected to some extent preoperatively, the clinical result was tolerable like the result of balloon test occlusion. A large aneurysm arising from A1 is extremely rare, and to our knowledge, this is the second case of large thrombosed A1 aneurysm treated endovascularly6). So we guess that this case was an exceptional aneurysm arising from A1.
In four cases of ruptured A1 aneurysms except large thrombosed one, the maximum diameter of these aneurysms was less than 6.2 mm. In a large number of aneurysm cases, the average diameter of the ruptured aneurysms was reported to be 8.2 mm9). The median diameter of our four SAH cases was 4.8 mm; significantly smaller than the majority of those reported in the literature. Wakabayashi et al.16) reported the average diameter in 8 ruptured A1 aneurysms was only 3.6 mm, which was as small as in our series. Thus, they emphasized that A1 aneurysms tend to rupture at a smaller size. We agree with the significance of this fact.
Besides this specific feature of the size, the literature notes several unique clinical characteristics of these aneurysms : frequency in young adolescents, in males or on the right side, fragility of the aneurysms, frequent ganglionic hemorrhage, vascular anomaly, fusiform shape and/or multiplicity of aneurysms7,8,16,17). Our series have similar tendency of previous reports. In our cases, there are four patients (36%) with multiple aneurysm, right side dominance (8 of 11 cases, 73%), and one patient (9%) with a vascular anomaly of accessory middle cerebral artery (Patient 2). Our series also had a higher rate of ganglionic hemorrhages among SAH because of ruptured A1 aneurysm (2 of 5 cases, 40%). There was no case of a fusiform aneurysm in our series. Dashti et al.5) reported 2 cases of fusiform aneurysm out of 23 A1 aneurysms. It was almost impossible to salvage all perforators due to the long segmental involvement of the fusiform aneurysm. Chang et al.3) advised segmental occlusion in that case rather than stent- or balloon-assisted endosaccular embolization to result in an excellent clinical outcome.
Even if a key point in the direct neck clipping of A1 aneurysms is to preserve the perforating arteries around the aneurysms, the key factors in the endovascular treatment of A1 aneurysm are effective and stable microcatheter tip positioning in the sac, and the coil insertion without kick-back of the microcatheter tip3,6,10,12). The complicated geometry of an aneurysm formed by the acute angle between the terminal segment of the ICA and the A1 segment makes it challenging to insert the microcatheter tip into the aneurysm sac6,13,14). Furthermore, if the microcatheter guidewire enter A1 origin, then it must immediately be turned in the opposite direction to enter the aneurysm. Even after successful selection of the aneurysm, the microcatheter is prone to kick back due to instability. Various adjunctive techniques have been introduced to overcome these complicated angio-architectures during the endovascular treatment. Lubicz et al.12) used balloon assisted coiling and/or a retrograde approach via the contralateral ICA through the anterior communicating artery in order to safely catheterize A1 aneurysms. But, this method might be restricted to some cases according to the condition of contralateral A1 or anterior communicating artery. Lee et al.10) reported a "Z-shaped" microcatheter tip was helpful in achieving stable microcatheter tip positioning for A1 aneurysms, especially located at the proximal portion of the A1. We could achieve complete occlusion in most cases, using the relevant combination of appropriate shaping of the microcatheter and double microcatheter technique. These methods permitted accurate positioning and support of microcatheter during the procedure, even though balloon was necessary in two cases.
The limitations of this study include its retrospective design, patient-selection bias, limited number of cases in one institution, and the relatively short angiographic follow-up period. Besides, more A1 aneurysms were clipped or not treated for various reasons, which may also have imposed a bias on the results. However, all these data suggest that endovascular treatment for A1 aneurysms seems to be feasible, safe, and effective.
Our preliminary experience demonstrates that endovascular treatment for A1 aneurysms is feasible and safe. Endovascular treatment appears to be a good therapeutic option for A1 aneurysms. These aneurysms are associated with multiple lesions and are less prone to recanalization because they are small and sidewall aneurysms. Tailored microcatheter shaping and/or adjunctive techniques are necessary to achieve successful aneurysm embolization. However, more adequate follow-up studies are required to evaluate its long-term results.
References
1. Barry DM. Surgical treatment of anterior cerebral-anterior communicating artery aneurysms. R I Med J. 1972; 55:245–247. PMID: 4506289.
2. Chalif DJ, Weinberg JS. Surgical treatment of aneurysms of the anterior cerebral artery. Neurosurg Clin N Am. 1998; 9:797–821. PMID: 9738108.

3. Chang HW, Youn SW, Jung C, Kang HS, Sohn CH, Kwon BJ, et al. Technical strategy in endovascular treatment of proximal anterior cerebral artery aneurysms. Acta Neurochir (Wien). 2011; 153:279–285. PMID: 20872259.

4. Czepko R, Libionka W, Lopatka P. Characteristics and surgery of aneurysms of the proximal (A1) segment of the anterior cerebral artery. J Neurosurg Sci. 2005; 49:85–95. PMID: 16288191.
5. Dashti R, Hernesniemi J, Lehto H, Niemelä M, Lehecka M, Rinne J, et al. Microneurosurgical management of proximal anterior cerebral artery aneurysms. Surg Neurol. 2007; 68:366–377. PMID: 17905060.

6. Gupta R, Horowitz MB, Gilman S. Neuroform stent-assisted coil embolization of a ruptured A1 segment anterior cerebral artery aneurysm. J Neuroimaging. 2006; 16:117–119. PMID: 16629732.

7. Handa J, Nakasu Y, Matsuda M, Kyoshima K. Aneurysms of the proximal anterior cerebral artery. Surg Neurol. 1984; 22:486–490. PMID: 6495158.

8. Hino A, Fujimoto M, Iwamoto Y, Oka H, Echigo T. Surgery of proximal anterior cerebral artery aneurysms. Acta Neurochir (Wien). 2002; 144:1291–1296. discussion 1296. PMID: 12478340.
9. Kassell NF, Torner JC. Size of intracranial aneurysms. Neurosurgery. 1983; 12:291–297. PMID: 6843800.

10. Lee HY, Ahn JS, Suh DC, Lee DH. Z-shaped microcatheter tip shaping for embolization of aneurysms at the proximal A1 segment of the anterior cerebral artery : a technical note. Neurointervention. 2011; 6:95–99. PMID: 22125756.

11. Lee JM, Joo SP, Kim TS, Go EJ, Choi HY, Seo BR. Surgical management of anterior cerebral artery aneurysms of the proximal (A1) segment. World Neurosurg. 2010; 74:478–482. PMID: 21492598.

12. Lubicz B, Bruneau M, Dewindt A, Lefranc F, Balériaux D, De Witte O. Endovascular treatment of proximal anterior cerebral artery aneurysms. Neuroradiology. 2009; 51:99–102. PMID: 18985332.

13. Rosner SS, Rhoton AL Jr, Ono M, Barry M. Microsurgical anatomy of the anterior perforating arteries. J Neurosurg. 1984; 61:468–485. PMID: 6747683.

14. Serizawa T, Saeki N, Fukuda K, Yamaura A. [Microsurgical anatomy of the anterior communicating artery and its perforating arteries important for interhemispheric trans-lamina terminalis approach : analysis based on cadaver brains]. No Shinkei Geka. 1994; 22:447–454. PMID: 8196831.
15. Suzuki M, Onuma T, Sakurai Y, Mizoi K, Ogawa A, Yoshimoto T. Aneurysms arising from the proximal (A1) segment of the anterior cerebral artery. A study of 38 cases. J Neurosurg. 1992; 76:455–458. PMID: 1738027.

16. Wakabayashi T, Tamaki N, Yamashita H, Saya H, Suyama T, Matsumoto S. Angiographic classification of aneurysms of the horizontal segment of the anterior cerebral artery. Surg Neurol. 1985; 24:31–34. PMID: 4012555.

17. Wanibuchi M, Kurokawa Y, Ishiguro M, Fujishige M, Inaba K. Characteristics of aneurysms arising from the horizontal portion of the anterior cerebral artery. Surg Neurol. 2001; 55:148–154. discussion 154-155. PMID: 11311909.

Fig. 1
Patient 10. Images of 42-year-old man with a ruptured large thrombosed aneurysm of right A1. A and B : Unsubtracted and subtracted images of conventional angiography shows a large aneurysm arising from mid-A1. Contrast retention (white arrow) and filling defect (black arrow) in the aneurysm imply a considerable amount of thrombus. C : Unsubtracted images acquired immediately after coiling reveals total occlusion of the aneurysm sac and the ipsilateral A1. D : Left internal carotid angiogram demonstrates sufficient flow of the right anterior cerebral artery provided through the anterior communicating artery.
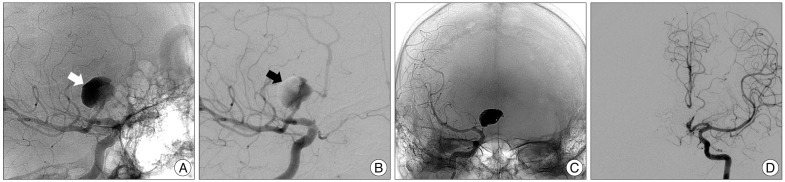
Fig. 2
Patient 11. Images showing a ruptured aneurysm of right A1. A : Computed tomographic examination from a 77-year old male patient with acute ganglionic hemorrhage. B : Conventional angiography shows a right small A1 aneurysm with a small neck. The aneurysm is superiorly located on the parent artery, just a few millimeters after the internal carotid artery bifurcation. C : Roadmap image shows that accurate aneurysm selection with S-shaped microcatheter (arrow) is done and guiding catheter is placed in petrous ICA as distal as possible. D and E : Unsubtracted and subtracted images acquired immediately after coiling reveal complete occlusion of aneurysm. F : The 6 month follow-up angiogram reveals stable occlusion of the aneurysm.
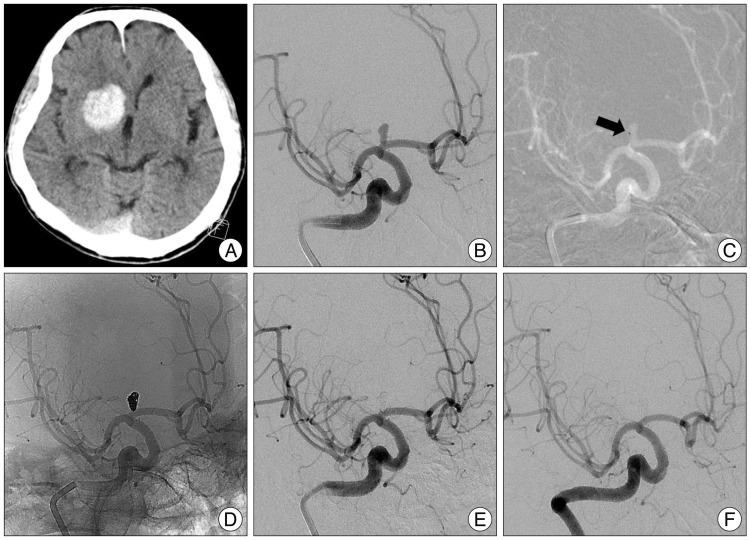
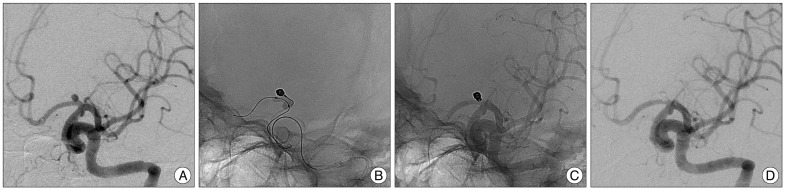
Fig. 3
Patient 1. Images of 47-year-old woman with an unruptured aneurysm of left A1. A : Diagnostic angiography demonstrates a posteriorly projecting saccular aneurysm arising at left proximal A1. Catheterization of aneurysm sac was achieved using a steam-shaped microcatheter in an "S-shape", but compact packing of the aneurysm was not possible due to early kick-back of the microcatheter. B : HyperForm balloon is positioned across the aneurysm neck to stabilize the microcatheter. And the first coil is inserted into the aneurysm with the balloon inflation and bridging the aneurysmal neck. C and D : Unsubtracted and subtracted images acquired immediately after coiling demonstrate dense, complete occlusion of the aneurysm without compromising the parent artery.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download