Abstract
Charcot spinal arthropathy is a rare, progressive type of vertebral joint degeneration that occurs in the setting of any preexisting condition characterized by decreased afferent innervation to the extent that normal protective joint sensation in the vertebral column is impaired. The authors report on a case of Charcot arthropathy of the lower lumbar spine mimicking a spinal tumor following cervical cord injury.
Charcot spinal arthropathy (CSA) is a rare, progressive vertebral joint degeneration that occurs in the setting of any preexisting condition characterized by decreased afferent innervation severe enough to impair the normal protective sensation of joints of the vertebral column1). Historically, CSA occurs most commonly in the setting of tertiary syphilis, but more contemporary CSA case series almost exclusively comprise patients with traumatic spinal cord injury1-5).
We report on a case of Charcot arthropathy of the lower lumbar spine mimicking a spinal tumor following cervical cord injury.
A 38-year-old man was admitted to our department with a one-month history of back pain radiating from the right flank and abdomen that had recently worsened. He had developed quadriplegia after spinal cord injury at C5-6 (Fig. 1). For last twenty years previously, he had been in bed ridden state. He had also experienced intermittent back pain for 10 years. The patient presented at our institute to check a lumbosacral osteolytic mass found by computed tomography (CT) at another institute during an investigation of growing back pain. Clinical examination showed a complete, flaccid, and areflexic paralysis of both lower limbs, with no sensation below T2. The patient had limitations of motion with no significant contractures at the right hip, and no palpable masses, fluid collections, or appreciable lymphadenopathy. Laboratory analysis revealed no signs of a spinal infection.
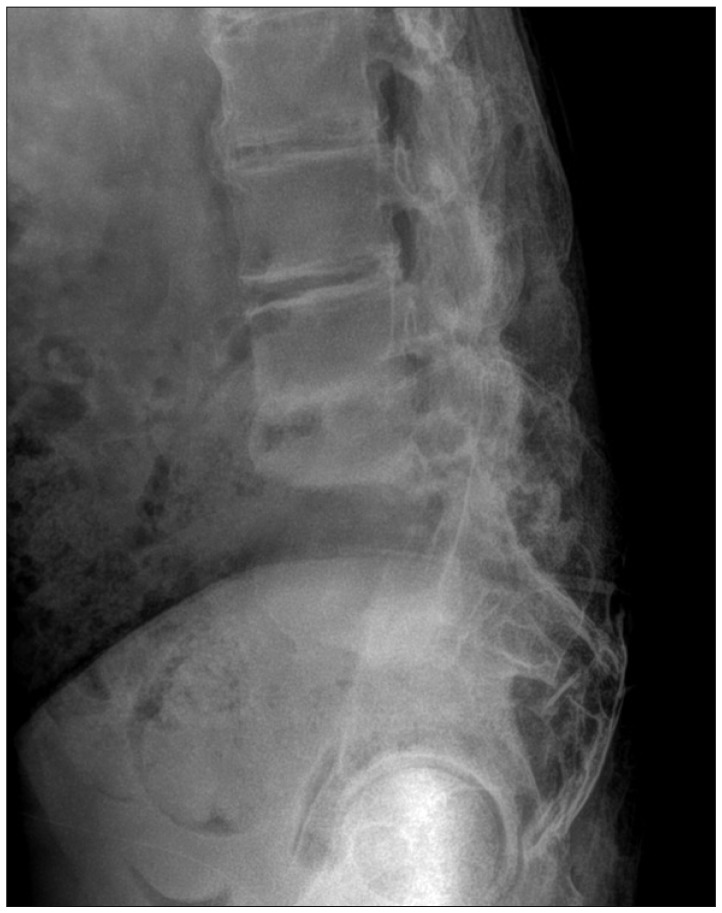
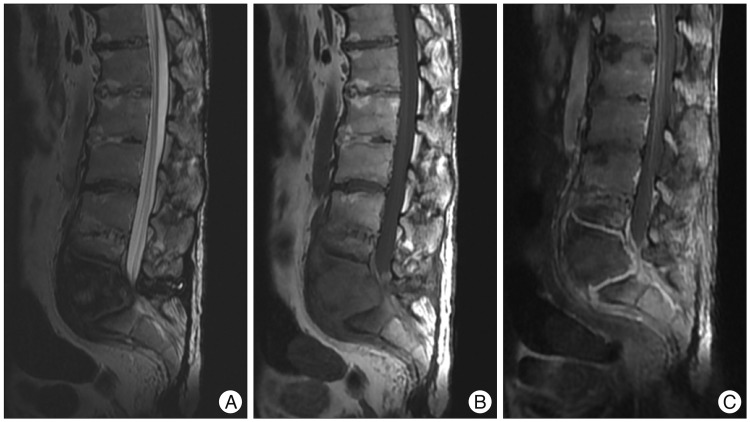
Plain radiography revealed erosion and destruction of L5 and the sacral vertebral body and the presence of paravertebral hypertrophic ossification with a pseudotumoral appearance around the L5-S1 joint (Fig. 2). A three dimensional CT scan highlighted the severity of the intervertebral dislocation in the lying position (Fig. 3), and magnetic resonance imaging (MRI) showed peripheral and paravertebral gadolinium uptake by the osteolytic lesion at L5 and S1 vertebrae (Fig. 4).
In view of these radiological features, the possibility of a chronic infection or a cancerous process was envisaged, and this prompted us to perform a vertebral needle biopsy. Cultures of central/disc liquid samples were negative, and histological findings revealed extensive fibrotic change but no evidence of an infective or neoplastic process. Based on clinical history, laboratory finding, and histologic features the lesion was consistent with CSA.
In terms of therapeutic options, the extensive spinal ankylosis and widespread para-osteoarthropathy around hips meant that stabilization surgery would probably have resulted in a poor functional outcome. Moreover, the patient refused a surgery because of the absence of malignancy. An external support with a body jacket molded for sitting was also suggested but the patient refused. Hence, we were only able to implement regular clinical and radiological monitoring. During follow-up no specific changes were observed after discharge.
Neuropathic spinal arthropathy is rare and was first described in 1868 by Jean-Martin Charcot in patients with tertiary syphilis1). However, any disease process that destroys deep joint sensation and leads to continued activity despite injury or inflammation within the joint, is capable of producing Charcot arthropathy5). Recently, the condition is encountered more commonly as a result of traumatic spinal injuries, spinal tumors, or syringomyelia, or secondary to diabetes1-5).
The mechanisms underlying the occurrence of Charcot spine include excessive loading of the thoracolumbar and lumbar spine during transfer activities in paraplegic patients coupled with impaired pain and proprioceptive sensations1-6). In paraplegic patients, spinal stress in the position sitting and during transfer is significant. Furthermore, the risk of the early occurrence of Charcot spine is greater in active spinal cord injured patients. Other mechanisms may include iatrogenic instability due to laminectomy and the concentration of loads on lower adjacent segments fused by previous operation6).
In cord injury patients with complete neurologic impairment, the most frequent symptoms of CSA are a feeling of instability in the sitting position and spinal deformity (usually with thoracolumbar gibbosity and an audible cracking noise)4,5). Spinal pain is also frequent, and may be mechanical or inflammatory in nature and sometimes difficult to differentiate from neuropathic pain. Changes in the neurological picture have also been described, such as, accentuated spasticity in partial paraplegics, decreased spasticity in complete paraplegics, and changes in bladder and bowel disorders. The time lag between the onset of neurological impairment and a diagnosis of CSA is usually long (17.3 years, on average), and in our case was about 20 years to diagnosis. The time lag between the first symptoms of CSA and its diagnosis is also long in many cases, due to the relatively non-specific nature of the apparent symptoms.
Uncommonly, CSA manifests as a paravertebral, pseudotumoral mass, as in our case. The differential diagnosis involves consideration of progressive spinal destructive lesions, such as, pyogenic spondylitis and metastatic spinal tumor. The diagnostic criteria for Charcot spine are as follows : 1) The presence of underlying disease causing impairment of deep pain sensation and proprioception. 2) Massive bone destruction and resorption coupled with equally profuse new bone formation on simple radiographs. 3) Histopathological findings of nonspecific chronic inflammation, which rules out other inflammatory diseases and tumorous conditions6).
The radiological aspects of the Charcot spine reflect the disease mechanism and usually combine the following features : 1) significant disc degeneration; 2) destruction/erosion of the vertebral body, associated with osteosclerosis or osteolysis, and occasionally, the presence of sequestra or debris resulting from fragmentation of subchondral bone; 3) hypertrophic osteophytosis within paravertebral soft tissue with a markedly pseudotumoral appearance; and 4) early-onset damage to facet joints1). CT is useful for assessing the severity of vertebral body bone destruction and the extent of paravertebral bone formation, whereas MRI frequent exhibits peripheral contrast uptake (attesting to the chronic inflammation that accompanies the disc and vertebral damage) and more occasionally, paravertebral gadolinium uptake.
Although natural progression of the disease is slow, it is clearly related to the aggravation of disc/vertebral lesions. It is also important to emphasize the risk of secondary neurological aggravation in incompletely paraplegic patients. The various therapeutic options consist of monitoring, immobilization with a body jacket, or surgery1). If patient's general conditions are favorable, the optimal treatment is surgical reduction of the deformity and permanent stabilization of the spine. Bone fusion should be achieved ideally by a posterolateral graft and an anterior graft using a combined approach or an extensive posterior approach2-6). However, indications for surgery must be discussed on a case-by-case basis according to clinical context (age, severity of spinal deformation, pain, etc.) and the potential functional impact of the surgical procedure. We considered our patient an inappropriate candidate for surgical management, given his relatively poor general health, the care goals of personal and family member, and the substantial bone destruction present at the time of diagnosis. Accordingly, we adopted a conservative treatment option.
We report a case of CSA and describe the main clinical, radiological, and therapeutic aspects of this rare and probably overlooked pathology. If patient's general conditions are favorable, surgical reduction of the deformity and permanent stabilization of the spine could be recommended. However, indications for surgery must be considered on a case-by-case basis according to clinical context and potential functional impact.
References
1. Barrey C, Massourides H, Cotton F, Perrin G, Rode G. Charcot spine : two new case reports and a systematic review of 109 clinical cases from the literature. Ann Phys Rehabil Med. 2010; 53:200–220. PMID: 20338837.
2. Chisholm KA, Gilchrist JM. The Charcot joint : a modern neurologic perspective. J Clin Neuromuscul Dis. 2011; 13:1–13. PMID: 22361621.
3. David KS, Agarwala AO, Rampersaud YR. Charcot arthropathy of the lumbar spine treated using one-staged posterior three-column shortening and fusion. Spine (Phila Pa 1976). 2010; 35:E657–E662. PMID: 20505559.

4. Proietti L, Pola E, Nasto LA, Scaramuzzo L, Logroscino CA. Onset of a Charcot spinal arthropathy at a level lacking surgical arthrodesis in a paraplegic patient with traumatic cord injury. Eur Spine J. 2010; 19(Suppl 2):S83–S86. PMID: 19504271.

5. Rose DM, Hilton AI, Tucker SK. Charcot spinal arthropathy in a paraplegic weight lifter : case report. Spine (Phila Pa 1976). 2006; 31:E339–E341. PMID: 16688025.
6. Suda Y, Shioda M, Kohno H, Machida M, Yamagishi M. Surgical treatment of Charcot spine. J Spinal Disord Tech. 2007; 20:85–88. PMID: 17285059.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download