Abstract
Ependymoma can spread via cerebrospinal fluid, but late spinal recurrences of intracranial tumor are very rare. We describe a case of a 33-year-old male who presented with multiple, delayed, recurrent lesions in the spinal cord from an intracranial ependymoma. The patient underwent gross total resection and postoperative radiation therapy 14 years prior to visit for a low grade ependymoma in the 4th ventricle. The large thoraco-lumbar intradural-extramedullary spinal cord tumor was surgically removed and the pathologic diagnosis was an anaplastic ependymoma. An adjuvant whole-spine radiation therapy for residual spine lesions was performed. After completion of radiation therapy, a MRI showed a near complete response and the disease was stable for three years.
Intracranial ependymomas constitute 3-5% of all primary intracranial tumors2,10). Intracranial ependymomas may spread by local infiltration or by dissemination via the cerebrospinal fluid (CSF). The precise incidence of cerebrospinal dissemination of ependymomas is unknown. However, high-grade tumors, infratentorial location, and incomplete resection at the primary site are known risk factors for dissemination. Dissemination of the tumor usually occurs within few years after the initial diagnosis and late spinal recurrences of intracranial low grade ependymoma are extremely rare1,3,5,8,9,14-16,18,19). We managed a patient with multiple spinal cord recurrences with anaplastic transformation from a low grade ependymoma in the fourth ventricle, 14 years after initial surgery without evidence of local failure.
33-year-old male visited with the complaint of sudden dysuria, difficulty in defecation, and progressive paraparesis. He also had a thoraco-lumbar back pain for two months. Bilateral weakness in the leg muscles (grade 4) and hypesthesia/numbness below T12 dermatome were found in the neurological examinations. Micturition was not possible. There were no specific abnormalities in the cranial nerves and upper extremities.
Fourteen years prior, the patient was diagnosed with a fourth ventricle low grade ependymoma. A gross total resection of the tumor with a ventriculoperitoneal shunt procedure was performed. Postoperative radiation therapy was also performed delivering 45 Gy to the posterior fossa and 10 Gy boost to the tumor bed. At initial surgery, the evaluation for spinal metastasis was not considered and prophylactic spinal irradiation was not performed because the intracranial ependymoma was low grade. Follow up was continued for three years, without any sign of tumor recurrences. All the previous medical records were meticulously reviewed.
The thoracolumbar MR image taken at a different hospital demonstrated an intradural-extramedullary mass involving T12 and L1 (Fig. 1). The tumor was 5.7 cm in size, dorsal in location, and was compressing the spinal cord. Emergency operation with a routine laminectomy of T12-L1 was performed. A gray-red intradural extramedullary mass was noted and was easily separated from the spinal arachnoid and completely removed without complication. Weakness and voiding difficulties experienced by the patient improved immediately after surgery.
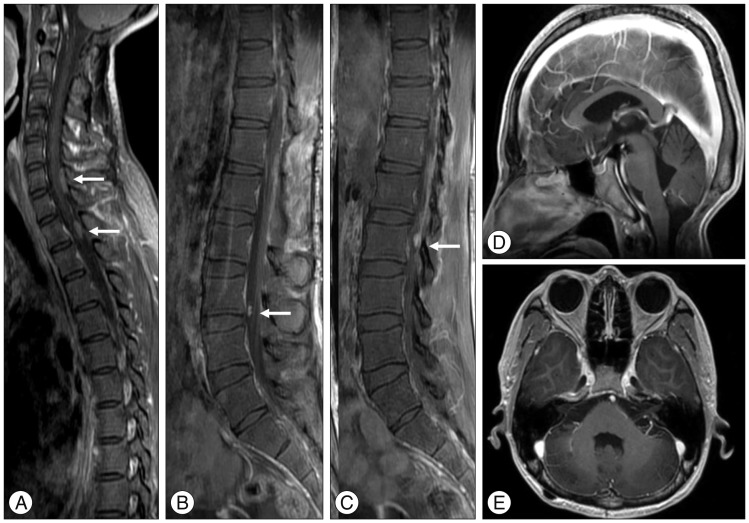
The histologic findings of the lesion were consistent with an ependymal tumor with a high Ki-67 mitotic index (up to 60%), moderate nuclear pleomorphism, and tumor necrosis. Based upon the histology, a diagnosis of World Health Organization (WHO) grade III anaplastic ependymoma was made (Fig. 2). A postoperative whole spine MR study revealed multiple small enhancing lesions from C6 to L5 (Fig. 3). A brain MR showed no evidence of residual tumor or tumor recurrence (Fig. 3). No other ventriculoperitoneal shunt pathway-related metastases were found.
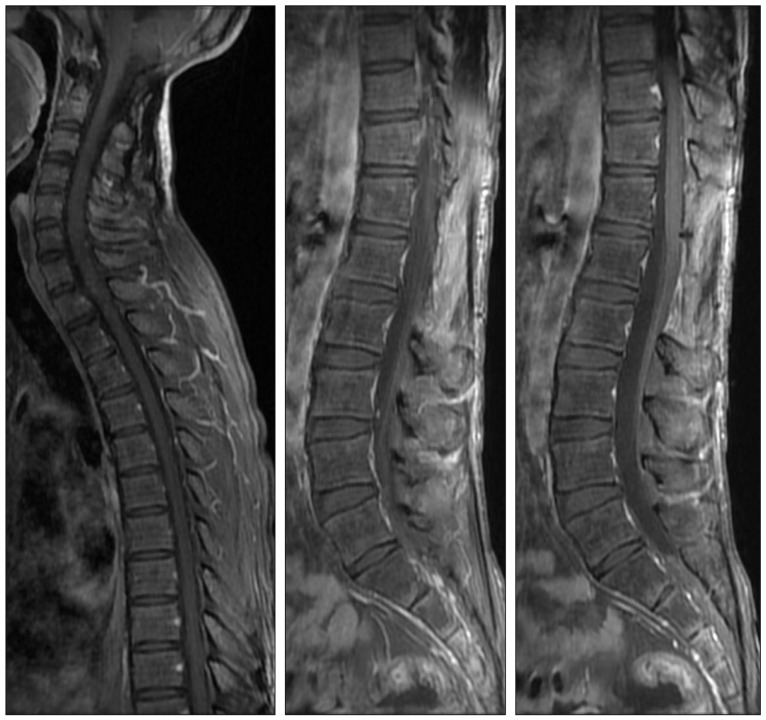
The patient received a radiation therapy to the whole spine of 36 Gy in 1.5 Gy fractions and with an additional boost of 9 Gy in 1.8 Gy fractions to the thoraco-lumbar tumor site using a 6 MV photon. During radiation therapy, RTOG/EORTC grade 2 hematologic toxicity was observed and resolved without specific treatment. Chemotherapy was reserved for disease progression. The patient's neurologic and clinical status improved and remained stable. Follow up images showed disappearance or a decrease in size of the spinal residual mass after radiation therapy and there was no evidence of tumor progression for three years (Fig. 4).
The proliferative and dissemination kinetics of ependymomas are unpredictable. Dissemination of low grade ependymoma is relatively rare, especially when a primary tumor is under control. Most common pattern of treatment failure is localized relapse. Metellus reported that only one patient showed CSF dissemination in 114 intracranial ependymoma patients, and according to Lyon's literature review, 3% of 219 patients with low-grade infratentorial ependymoma developed spinal seeding11,13). The true incidence of dissemination may be higher because follow up studies of the spinal cord are often neglected when local tumors are controlled1).
In this case, the spinal cord tumors were thought to be disseminated rather than de novo. Because spinal cord ependymomas usually arise from ependymal cells, de novo ependymomas usually appear as intramedullary tumors (except for those occurring in the conus medullaris and filum terminale)15). Intradural extramedullary primary spinal cord ependymomas were reported, which is exceptional8). We did not note the invasion of arachnoid plane of the spinal cord in the surgical field and the other residual tumors appeared to be located extramedullary in the image studies. These findings support a diagnosis of fourth ventricle ependymoma with spinal metastasis.
Low grade ependymomas may possibly recur later because of their slow growth5). The median time to recurrence is 18 months for grade III ependymomas but Chao reported that the median time to recurrence is 7.7 years for benign spinal myxopaillary ependymomas4,13). Delayed recurrence may be considered as one of the main characteristics of low grade ependymomas1). Patients with low grade ependymomas should be followed longer than patients with anaplastic tumors.
There are only three reports presenting late (over 10 years) spinal cord recurrences of the 4th ventricular ependymomas thus far, to the best of our knowledge1,14,15). These reported cases and ours, show similar clinical features; low grade fourth ventricular ependymoma, gross total removal of primary lesion, subsequent radiation therapy to posterior fossa, and no evidence of local failure. The recurrent disseminated lesions were treated with surgical resection and/or whole spine radiation. We could postulate that 'late' spinal dissemination or recurrence may be a characteristic feature only observed in low-grade ependymomas, as Ochiai described15). Early detection of the dissemination of low grade ependymoma during the asymptomatic stage may prolong survival1,6). In patients with cranial or spinal ependymomas, even low grade, a MRI of the entire neuraxis is necessary to exclude metastases at distant sites.
A unique feature of our case is delayed anaplastic transformation of low grade ependymoma. This is exceptionally rare and up to our knowledge, this is the first case report of recurrence with anaplastic transformation in the spinal cord from low grade 'intracranial' ependymoma. There is one report in the literature of a patient with an extirpated benign intradural extramedullary ependymoma at the thoracic level with a remote recurrence that unexpectedly showed anaplastic transformation17). The mechanism of malignant transformation in ependymoma is not explained. Molecular genetic studies have not been useful in recognizing anaplastic features in ependymomas at present2).
A plausible explanation of the recurrence in our case is that the benign ependymoma disseminated to the spinal cord via the CSF at the time of primary diagnosis, and remained was dormant for a long time. The occult tumor underwent an anaplastic transformation, and thereafter, anaplastic ependymoma cells may have migrated with the CSF circulation leading to the formation of multiple intradural-extramedullary metastasis.
Another characteristic feature of this case is that the disseminated anaplastic tumor was controlled successfully by radiotherapy and secondary recurrence was inhibited for three years. In similar cases of late spinal dissemination, the surgery and whole spine radiotherapy was combined in one case, only whole spine radiation was performed in one case, and only gross total resection was done in one case. There were no descriptions of long term outcome in these patients. The patient in our case was neurologically stable, and the disseminated tumor were disappeared or decreased in size in follow-up MR images for relatively long period even with malignant pathological features.
The role and effectiveness of radiotherapy in the treatment of spinal cord ependymomas is still a matter of debate3,12,13,20). Especially for the spinal anaplastic ependymomas or recurrent tumors, there are no large studies to confirm the role of radiation owing to the low incidence. It is obvious that radiation had an effective role in controling anaplastic, recurrent and disseminated tumors in this case. Additional cases with a carefully controlled study are necessary to confirm the effectiveness of adjuvant radiation in patients with recurrent ependymomas.
The patient has had a gadolinium enhanced MRI of the whole spine annually. As ten-year progression free survival is 75% in spinal ependymomas, follow-up for a minimum 10 years would be necessary7).
References
1. Bademci G, Tun K, Erden E, Evliyaoglu C, Unlu A. Late dissemination of ependymoma : case report. Neurocirugia (Astur). 2007; 18:333–336. PMID: 17882342.
2. Bortolotto S, Chiadò-Piat L, Cavalla P, Bosone I, Mauro A, Schiffer D. CDKN2A/p16 in ependymomas. J Neurooncol. 2001; 54:9–13. PMID: 11763427.
3. Celli P, Cervoni L, Salvati M, Cantore G. Recurrence from filum terminale ependymoma 42 years after 'total' removal and radiotherapy. J Neurooncol. 1997; 34:153–156. PMID: 9210062.
4. Chao ST, Kobayashi T, Benzel E, Reddy CA, Stevens GH, Prayson RA, et al. The role of adjuvant radiation therapy in the treatment of spinal myxopapillary ependymomas. J Neurosurg Spine. 2011; 14:59–64. PMID: 21142463.

5. Ernestus RI, Schröder R, Stützer H, Klug N. The clinical and prognostic relevance of grading in intracranial ependymomas. Br J Neurosurg. 1997; 11:421–428. PMID: 9474274.

6. Good CD, Wade AM, Hayward RD, Phipps KP, Michalski AJ, Harkness WF, et al. Surveillance neuroimaging in childhood intracranial ependymoma : how effective, how often, and for how long? J Neurosurg. 2001; 94:27–32. PMID: 11147894.
7. Halvorsen CM, Kolstad F, Hald J, Johannesen TB, Krossnes BK, Langmoen IA, et al. Long-term outcome after resection of intraspinal ependymomas : report of 86 consecutive cases. Neurosurgery. 2010; 67:1622–1631. discussion 1631. PMID: 21107192.

8. Iunes EA, Stávale JN, de Cássia Caldas Pessoa R, Ansai R, Onishi FJ, de Paiva Neto MA, et al. Multifocal intradural extramedullary ependymoma. Case report. J Neurosurg Spine. 2011; 14:65–70. PMID: 21142461.
9. Kim DG, Cho BK, Yang HJ, Chi JG, Jung HW, Kim HJ, et al. Intracranial Ependymoma : Clinicopathologic Features and Prognostic Factors. J Korean Neurosurg Soc. 1991; 20:893–899.
10. Kleihues P, Burger PC, Scheithauer BW, Zülch KJ. Histological typing of tumours of the central nervous system, ed 2. Berlin: Springer-Verlag;1993.
11. Lyons MK, Kelly PJ. Posterior fossa ependymomas : report of 30 cases and review of the literature. Neurosurgery. 1991; 28:659–664. discussion 664-665. PMID: 1876243.
12. McLaughlin MP, Marcus RB Jr, Buatti JM, McCollough WM, Mickle JP, Kedar A, et al. Ependymoma: results, prognostic factors and treatment recommendations. Int J Radiat Oncol Biol Phys. 1998; 40:845–850. PMID: 9531369.

13. Metellus P, Guyotat J, Chinot O, Durand A, Barrie M, Giorgi R, et al. Adult intracranial WHO grade II ependymomas : long-term outcome and prognostic factor analysis in a series of 114 patients. Neuro Oncol. 2010; 12:976–984. PMID: 20484442.

14. Nakasu S, Ohashi M, Suzuki F, Matsuda M. Late dissemination of fourth ventricle ependymoma : a case report. J Neurooncol. 2001; 55:117–120. PMID: 11817702.
15. Ochiai H, Yamakawa Y, Kawano H, Shimao Y, Hayashi T. Late spinal cord metastasis of fourth ventricle ependymoma appeared nineteen years after the initial treatment. J Neurooncol. 2010; 96:295–299. PMID: 19629395.

16. Rehman S, Brock C, Newlands ES. A case report of a recurrent intracranial ependymoma treated with temozolomide in remission 10 years after completing chemotherapy. Am J Clin Oncol. 2006; 29:106–107. PMID: 16462515.

17. Rezai AR, Woo HH, Lee M, Cohen H, Zagzag D, Epstein FJ. Disseminated ependymomas of the central nervous system. J Neurosurg. 1996; 85:618–624. PMID: 8814165.

18. Salazar OM, Castro-Vita H, VanHoutte P, Rubin P, Aygun C. Improved survival in cases of intracranial ependymoma after radiation therapy. Late report and recommendations. J Neurosurg. 1983; 59:652–659. PMID: 6886786.

19. Vanuytsel L, Brada M. The role of prophylactic spinal irradiation in localized intracranial ependymoma. Int J Radiat Oncol Biol Phys. 1991; 21:825–830. PMID: 1831193.

20. Whitaker SJ, Bessell EM, Ashley SE, Bloom HJ, Bell BA, Brada M. Postoperative radiotherapy in the management of spinal cord ependymoma. J Neurosurg. 1991; 74:720–728. PMID: 2013772.

Fig. 1
Pre-operative outside thoracolumbar MR study showing a well demarcated and heterogeneously enhancing intradural extramedullary mass at the T12-L1 level. The spinal cord is considerably compressed by the tumor.
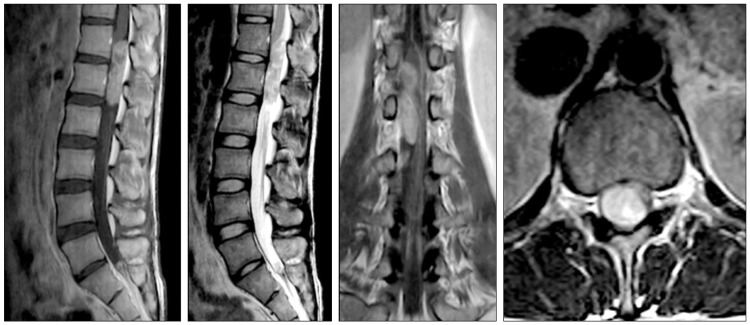
Fig. 2
Pathological study (A and B : H&E ×400, C and D : electron microscope) shows a solid tumor with neoplastic cells with round or elongated nuclei. Periluminal ciliary basal bodies and even clusters of blepharoplasts are noted, which is consistent with an anaplastic ependymoma.
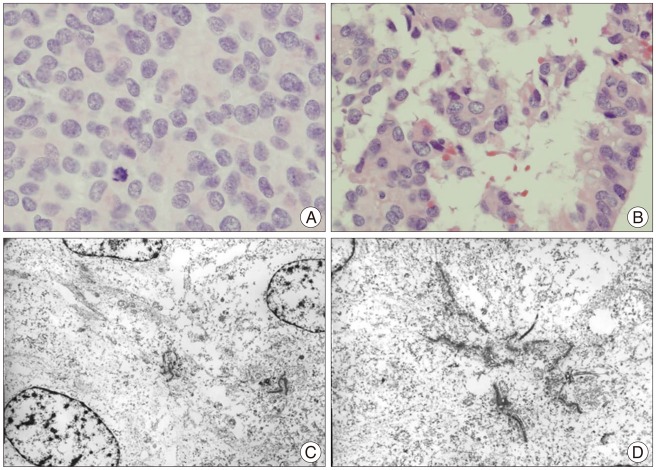
Fig. 3
The postoperative whole spine MR image demonstrats that the main thoracolumbar tumor has been removed but multiple intradural extramedullary enhancing lesions (arrows) from C6 to L5 are found (A, B, and C). There is no evidence of local recurrence of the tumor in the posterior fossa (D and E).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download