Abstract
Isolated symptomatic occlusion of the anterior cerebral artery (ACA) is a rare condition and until date, only few cases regarding the revascularization of the ACA have been reported. This paper reports on successful attempt to revascularize the ACA using superficial temporal artery (STA) in patient with isolated symptomatic occlusion of the ACA. A 69-year-old man presented with several episodes of transient weakness involving left lower extremity. Cerebral angiography showed occlusion of the right ACA at the A2 segment. After medical treatment failure, the patient underwent STA-ACA bypass surgery. Subsequent to surgery, there was immediate disappearance of transient ischemic attack and follow-up angiography showed favorable revascularization of the ACA territory. Bypass surgery can be considered in the patients with symptomatic occlusion of the ACA, who have experienced failure in medical treatment.
Isolated occlusion of the anterior cerebral artery (ACA) accompanied by cerebral infarct of the ACA territory is of rare occurrence. Furthermore, in patients with occlusion of the ACA, ischemic symptoms rarely occur because the territory of ACA receives collateral flow from many sources.
Although few cases of successful revascularization for symptomatic occlusion of the ACA have been reported3-6,9), controversy remains concerning the effectiveness of cerebral revascularization procedures for the prevention of strokes because of its rarity.
Herein, we describe a case of symptomatic occlusion of ACA in a 69-year-old man, and report on successful attempt to revascularize the ACA.
A 69-year-old man, with hypertension and past history as heavy smoker and chronic alcoholic for 40 years, presented with several episodes of transient weakness involving left lower extremity that lasted for 6 months. Each episode occurred once a week, and lasted approximately for 10-20 seconds. The initial magnetic resonance imaging (MRI) from another hospital revealed multifocal stenosis of cerebral artery including left proximal internal carotid artery (ICA), right proximal external carotid artery (ECA), right middle cerebral artery (MCA) at the M1 segment, and occlusion of right ACA at the A2 segment without any evidence of cerebral infarct in the diffusion weighted imaging. Despite the medical treatment with warfarin for one month, there was an increase in the frequency of transient ischemic attack (TIA) in the left leg. Follow-up MRI revealed acute focal infarct in the right frontal periventricular white matter corresponding to the territory of right ACA (Fig. 1). The rate of occurrence of TIA was once or twice a day despite combined medical treatment with warfarin and aspirin.
The patient visited our hospital one month after the first experience of infarct. On presentation, no apparent neurological deficit was found. Cardiac evaluation did not reveal any remarkable findings. Cerebral angiography revealed multifocal atherosclerotic luminal irregularity in both the ICA, especially moderate stenosis of the right supraclinoid ICA segment. Right ACA was occluded at the A2 segment, but collateral flow from right MCA and posterior cerebral artery (PCA) was minimal (Fig. 1). Basal and acetazolamide-stressed brain perfusion single photon emission computerized tomography (SPECT) with 99mTc-hexamethylpropyleneamin oxime was performed to evaluate the patient's hemodynamic status. Brain SPECT showed decreased perfusion and decreased vascular reserve in the right frontal lobe (Fig. 1).
Oral anticoagulant and antiplatelet agent were discontinued 1 week prior to surgery. After 2 months from experience of cerebral infarct, revascularization surgery was performed. After confirmation of sufficient length and integrity of parietal branch of the STA in preoperative ECA angiography, we planned end-to-side anastomosis between ipsilateral STA and distal ACA. Under general anesthesia, curvelinear skin incision was made along the parietal branch of the superficial temporal artery (STA), and then extended to the contralateral frontal just behind the hairline. The parietal branch of the STA were saved and prepared for anastomosis to the distal ACA. The frontal branch of the STA with galeal flap was saved as well during the preparation in case of adverse events, such as graft failure (Fig. 2). The length of parietal branch of the STA was 11 cm from the bifurcation of the STA. After right paramedian craniotomy and opening of the dura, the cortical branches of the right ACA were observed. We chose one of the cortical branches as a recipient artery, in which no flow was detected on the intraoperative Doppler. After confirmation of sufficient length and good pulsation of the parietal branch of the STA, end-to-side anastomosis between STA and ACA was performed (Fig. 2). Anterograde flow from the STA and flow diversion to the recipient artery was confirmed on the intraoperative Doppler.
The TIA of the left leg disappeared immediately after surgery. Oral antiplatelet agent (aspirin) was resumed on the first postoperative day with a dose of 100 mg/day. The patient was discharged with no apparent neurological deficit. Six months after the operation, follow-up cerebral angiography showed obvious flow through right STA to the territory of the right ACA and decreased leptomeningeal collateral flow from right PCA (Fig. 3). Brain SPECT revealed improved perfusion and vascular reserve when compared with preoperative imaging (Fig. 3). The patient received follow-up care for 30 months after surgery without occurrence of any remarkable events.
Cerebral infarcts localized in the anterior cerebral artery (ACA) territory are relatively rare, representing 0.5-3% of all ischemic strokes8). Gacs et al.2) reviewed computed tomography (CT) scans from 413 patients with ischemic infarction. In this study, 13 of 413 patients (3%) were confirmed to have CT evidence of infarct in the ACA territory. Among these 13 patients, occlusion of the ACA was found in only 8 patients (1.9%). Although the etiology of the ACA occlusion is poorly defined, large artery atherothrombosis, cardioemboli, and vasospasm are considered as major mechanisms of ACA occlusion1,2,7,8). In our case, the patient had risk factors such as hypertension, past history of heavy smoking and chronic alcoholic, which may have contributed to atherothrombosis and occlusion of the ACA. In patients with occlusion of the ACA, ischemic symptoms rarely occur because the territory of ACA, especially in the midline motor cortex for the legs, receives collateral flow from many sources. These include the ipsilateral ACA, the contralateral ACA through the anterior communicating artery, a leptomeningeal anastomosis between the ACA and the MCA or PCA, and also the posterior pericallosal artery3). Nevertheless, in our case, preoperative angiography and SPECT showed insufficient collateral flow from ipsilateral MCA and PCA to supplement the ACA territory, which resulted in TIA of lower extremity.
Despite the medical treatment with antiplatelet and anticoagulant in other hospital, the symptom was aggravated and new infarct was developed in ACA territory. Although controversy remains concerning the effectiveness of cerebral revascularization procedures for the patients with ACA occlusion, these findings suggest that bypass surgery rather than medical treatment may be helpful in early stages in patients with flow related symptomatic manifestations corresponding to the ACA territory, and unstable hemodynamics proven by SPECT.
Surgical revascularization is commonly performed in moyamoya disease (MMD), and most procedures are usually addressed to the MCA territory. Revascularization in the MCA territory might result in improvement of the hemodynamic status in the ACA territory, because there are leptomeningeal anastomoses between these territories. However, in MMD with poor leptomeningeal anastomoses between the ACA-MCA and the ACA-posterior cerebral artery, surgical revascularization to the ACA territory should be considered. These procedures include indirect and direct bypass surgeries. Generally, indirect procedures are effective in young patients with MMD. However, their effectiveness is limited in adult patients with atherosclerotic disease.
Several types of direct bypass surgery can be considered for revascularization of the ACA. Ito5) and Ikeda et al.3) performed side-to-side anastomosis between the distal ACA in patients with ACA occlusion. Side-to-side anastomosis seems to be a good procedure for utilizing the anatomic characteristics of the ACA. However, for this procedure to be successful, the contralateral ACA should be free from atherosclerosis, since occlusion of the anastomotic site might compromise the contralateral ACA, resulting in paraparesis9). Moreover, even if the patency of the anastomosis is preserved, blood flow distal to the anastomosed site on the normal side will nearly always decrease due to the steal phenomenon to the lesion side3). Therefore, there should be strict criteria for the use of this procedure in atherosclerotic disease presented with ischemic symptoms.
Ishii et al.4) reported on anastomosis of the STA to the distal ACA with interposed cephalic vein graft in patients with MMD. Iwata et al.6) reported interposed arterial graft bypass using the contralateral STA. Since there is no suitable scalp artery long enough to reach the territory of the ACA in most patients, several types of free grafts, such as the cephalic vein, saphenous vein, contralateral STA, and radial artery, can be used3-6,9). Although these procedures have advantages to choose and prepare the graft in a variety of situations, size discrepancy at the anastomosis site and kinking or torsion of the graft can cause graft failure. In addition, insufficient perfusion in the ACA territory might still remain even after successful anastomosis.
In our case, after confirming that the length of the ipsilateral STA was enough, we conducted STA-ACA anastomosis without using a graft. This type of bypass surgery, which achieved an excellent outcome in our patient, is not technically demanding, and requires a single anastomosis with less intraoperative time. If the length of STA is long enough to reach the territory of the ACA, as in this case, this type of bypass surgery can be considered as the primary surgical option. However, in case of scalp artery not long enough to reach the ACA, other type of bypass surgeries, such as STA-ACA anastomosis with interposed graft or side-to-side anastomosis between the ACA, is recommended.
We report a rare case of symptomatic occlusion of the ACA, and successful attempt to revascularize the ACA. When conducted in a timely fashion, bypass surgery could produce an excellent clinical outcome. Revascularization surgery should be considered as a treatment option in patients with symptomatic occlusion of the ACA.
References
1. Arboix A, García-Eroles L, Sellarés N, Raga A, Oliveres M, Massons J. Infarction in the territory of the anterior cerebral artery : clinical study of 51 patients. BMC Neurol. 2009; 9:30. PMID: 19589132.
2. Gacs G, Fox AJ, Barnett HJ, Vinuela F. Occurrence and mechanisms of occlusion of the anterior cerebral artery. Stroke. 1983; 14:952–959. PMID: 6659000.

3. Ikeda A, Okada T, Shibuya M, Noda S, Sugiura M, Iguchi I, et al. Revascularization of the anterior cerebral artery. Report of two cases. J Neurosurg. 1985; 62:603–606. PMID: 3973733.
4. Ishii R, Koike T, Takeuchi S, Ohsugi S, Tanaka R, Konno K. Anastomosis of the superficial temporal artery to the distal anterior cerebral artery with interposed cephalic vein graft. Case report. J Neurosurg. 1983; 58:425–429. PMID: 6827332.

5. Ito Z. [A new technique of intracranial interarterial anastomosis between distal anterior cerebral arteries (ACA) for ACA occlusion and its indication (author's transl)]. Neurol Med Chir (Tokyo). 1981; 21:931–939. PMID: 6171745.

6. Iwata Y, Mizuta T, Takemoto O, Shimizu K, Nakatani S. An interposed superficial temporal artery graft bypass for anterior cerebral artery ischemia. Microsurgery. 1988; 9:14–17. PMID: 3393069.

7. Kumral E, Bayulkem G, Evyapan D, Yunten N. Spectrum of anterior cerebral artery territory infarction : clinical and MRI findings. Eur J Neurol. 2002; 9:615–624. PMID: 12453077.

8. Sato S, Toyoda K, Matsuoka H, Okatsu H, Kasuya J, Takada T, et al. Isolated anterior cerebral artery territory infarction : dissection as an etiological mechanism. Cerebrovasc Dis. 2010; 29:170–177. PMID: 19955742.

9. Terasaka S, Satoh M, Echizenya K, Murai H, Fujimoto S, Asaoka K. Revascularization of the anterior cerebral artery using a free superficial temporal artery graft : a case report. Surg Neurol. 1997; 48:164–169. discussion 169-170. PMID: 9242243.

Fig. 1
Initial MRI, cerebral angiography, and SPECT of a 69-year-old man. MRI shows acute infarct in the right frontal periventricular white matter (A). Cerebral angiography reveals occlusion of right ACA at the A2 segment with insufficient filling of the right ACA territory (B), and minimal collateral flow from right PCA (C). Basal and acetazolamide-stressed SPECT shows decreased perfusion (D) and vascular reserve (E) in right frontal lobe. SPECT : single photon emission computerized tomography, ACA : anterior cerebral artery, PCA : posterior cerebral artery.
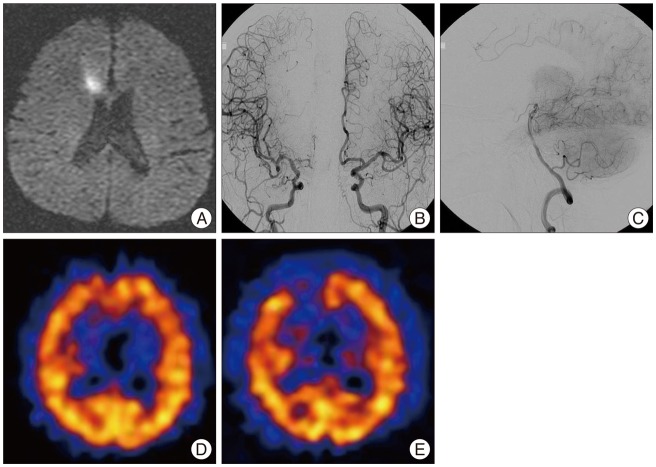
Fig. 2
Intraoperative photograph illustrating the preparation of the right STA (arrow) (A), and anastomosis between cortical branch of the ACA and parietal branch of the STA (B). ACA : anterior cerebral artery, STA : superficial temporal artery.
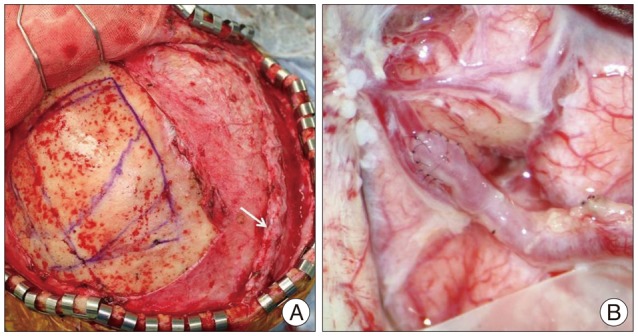
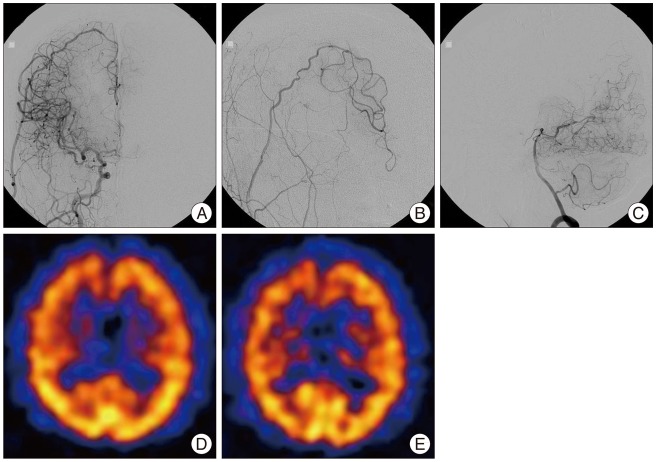
Fig. 3
Cerebral angiography and brain SPECT obtained on the sixth postoperative months. Cerebral angiography reveals anterograde flow through right STA to the territory of the right ACA (A : anteroposterior view, B : oblique view) and decreased leptomeningeal collateral flow from right PCA (C). Basal and acetazolamide-stressed SPECT shows improved perfusion (D) and vascular reserve (E) upon comparison with preoperative imaging. SPECT : single photon emission computerized tomography, STA : superficial temporal artery, ACA : anterior cerebral artery, PCA : posterior cerebral artery.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download