Abstract
Objective
Spinal cord stimulation (SCS) is an effective means of treatment of chronic neuropathic pain from failed back surgery syndrome (FBSS). Because the success of trial stimulation is an essential part of SCS, we investigated factors associated with success of trial stimulation.
Methods
Successful trial stimulation was possible in 26 of 44 patients (63.6%) who underwent insertion of electrodes for the treatment of chronic pain from FBSS. To investigate factors associated with successful trial stimulation, patients were classified into two groups (success and failure in trial). We investigated the following factors : age, sex, predominant pain areas (axial, limb, axial combined with limbs), number of operations, duration of preoperative pain, type of electrode (cylindrical/paddle), predominant type of pain (nociceptive, neuropathic, mixed), degree of sensory loss in painful areas, presence of motor weakness, and preoperative Visual Analogue Scale.
Results
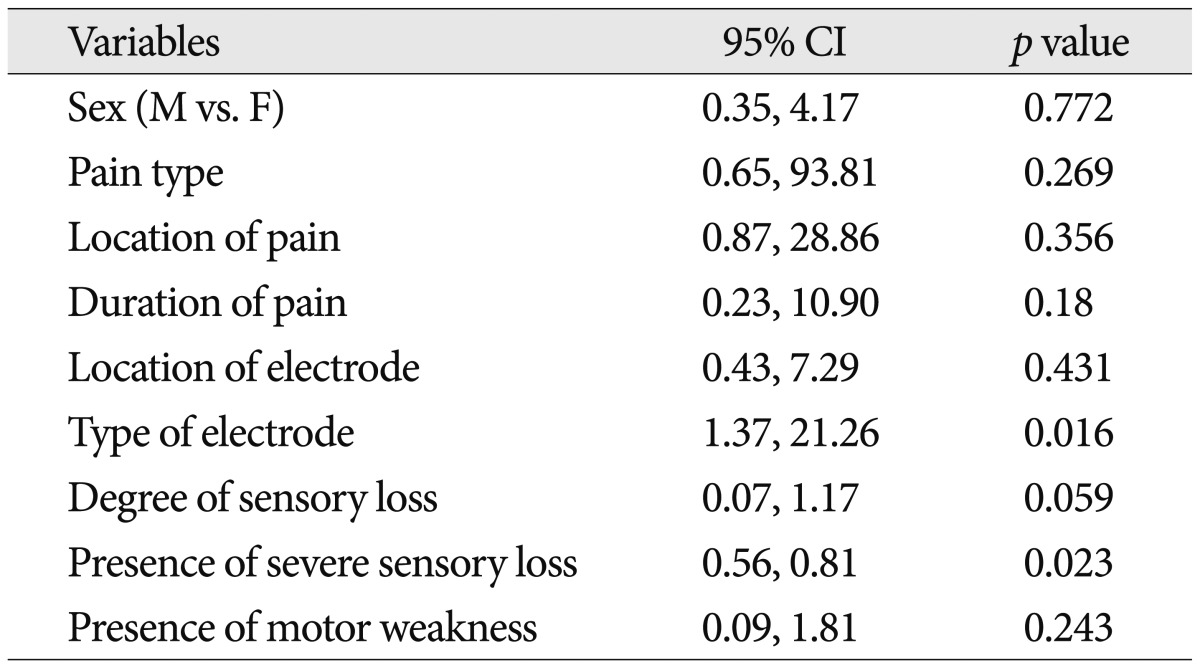
There were no significant differences between the two groups in terms of age, degree of pain, number of operations, and duration of pain (p>0.05). Univariate analysis revealed that the type of electrode and presence of severe sensory deficits were significantly associated with the success of trial stimulation (p<0.05). However, the remaining variable, sex, type of pain, main location of pain, degree of pain duration, degree of sensory loss, and presence of motor weakness, were not associated with the trial success of SCS for FBSS.
Failed back surgery syndrome (FBSS) is a challenging clinical entity for both patients who suffer from persistent pain and impaired function and clinicians who try their best to manage them12). Treatment of FBSS is challenging because medical treatment and repeated back surgeries are often unsuccessful in providing adequate pain relief. Despite the advances in surgical techniques, the rate of FBSS has remained similar for several decades8). Moreover, some evidence points to excessive rates of spine surgery in our country. Unfortunately, the failure rate of spinal surgery has not changed in the past several decades18,19,25). With the increasing rate of spinal surgery, the number of patients with FBSS has also increased6). It is also known that the success rate falls if subsequent operations are performed24).
Patients diagnosed with FBSS should be managed in an interdisciplinary environment12). When all conservative treatment fails, interventional therapies such as epidural adhesiolysis and spinal cord stimulation (SCS) are the recommended treatment options with accepted evidences12). SCS is known to be a safe and effective treatment option for selected patients with medically refractory chronic pain syndrome such as FBSS or complex regional pain syndrome (CRPS)1,3,6,7,10,16,17,21,26,35,37). SCS systems are routinely implanted after a successful screening trial. Although many studies have reported the results of SCS for patients with FBSS1,3,6,7,10,16,17,21,26,35,37), studies regarding factors associated with successful screening trial are rare. We retrospectively investigated factors associated with the success of trial stimulation for patients with FBSS.
Medical records of 44 patients who underwent insertion of SCS electrodes for the treatment of chronic pain from FBSS were reviewed. The mean age of the patients was 59.32±11.6 (mean±standard deviation), 16 of whom were female. All patients had chronic pain which developed after operation for degenerative spinal disorders. Table 1 summarizes the demographics of the patients with screening trials of SCS in our series.
Patients who underwent SCS trials had been refractory to previous medical treatments including analgesics, opioid analgesics, physical therapy, and pain blocks. SCS was considered for patients with a minimum Visual Analogue Scale (VAS) score of 7/10. Candidates with psychopathological or substance abuse problems and those with significant unresolved issues of secondary gain and worker's compensation were excluded in this study.
Candidates were admitted to the hospital for a trial of SCS. In 50% of these patients (22 of 44), a quadripolar electrode [Pisces™ (model 3487A), Medtronic Inc., Minneapolis, MN, USA, Quattrode® (model 3186), Octtrode® (model 3156), St. Jude Medical, Plano, TX, USA] were inserted with the patient under local anesthesia via a Touhy needle placed into the epidural space. The electrode was positioned to provide stimulation paresthesia overlapping the topography of the patient's area of pain. In the remaining patients (22 of 44, 50%), a small laminectomy was performed and a paddle electrode [Lamitrode® 44, Tripole 8, Tripole 16, St. Jude Medical, Plano, TX, USA and Resume® TL, Specify™ (model 3998), Specify™ 5-6-5 (model 39565), Medtronic Inc., Minneapolis, MN, USA] was placed (Fig. 1).
After implantation, the electrode was connected to a handheld programmer that allowed various levels of stimulation to be tested during a 3 to 7-day trial period. Patient's pain levels during trial stimulation were monitored using 0 to 10 digital scale. All narcotic medications for chronic pain were withheld during the trial period. Patients were encouraged to increase their activity levels to near normal levels after postoperative day one, so their reports of pain reduction could be a more accurate reflection of the extent to which the permanent stimulating system is expected to control the pain. After at least 50% reduction in pain, an implantable pulse generator (IPG) was implanted.
As part of physical examination, a thorough history of pain complaint and associated treatments was obtained. Before the SCS trial, the patients gave informed consent and answered a series of questions to determine their demographic and clinical history. Areas probed included age, sex, location of predominant pain (axial, unilateral and bilateral limb, combined back and limb), duration of pain, number of prior back surgeries, predominant type of pain (neuropathic, nociceptive, mixed), severity of pain at the time of SCS (VAS), presence of motor weakness, presence of sensory loss and significant sensory loss in painful areas, type of electrode used for the trial (cylindrical or paddle), and level of electrode (T8/9 or T12/L1, cervical).
All statistical analyses were performed using SPSS (version 15.0, SPSS Inc., Chicago, IL, USA). Exploratory analyses of the demographic and medical data were performed by calculating the means and standard deviations for continuous outcomes and
cross-tabulation for categorical measures. Chi-square tests were used to assess statistical differences between the two groups (trail success and trial failure) for categorical outcome variables. Statistical differences between the two groups were assessed using a two-sided t-test for independent samples. Logistic regression analysis was used for univariate analysis of variables associated with successful trial stimulation. Statistical significance was accepted at a probability value of less than 0.05.
Forty-four patients fit the inclusion criteria. As shown in Table 1, the study group consisted of 18 males and 26 females who underwent SCS for FBSS. The mean age was 59.3 years (11.6), and the duration of pain at the time of SCS was 72.36 months (87.74). The number of operation before SCS was 1.79 (0.90), and their mean preoperative VAS was 76.91 (10.26).
About 12% of FBSS patients had pain syndromes affecting their axial area (back and neck), while 50% of patients predominantly had extremity pain. About 50% of patients showed typical symptoms and signs of neuropathic pain according to the DN4 criteria5). Fifty percent of patients underwent a trial of SCS with cylindrical electrodes and the remaining 50% of the patients were tried with paddle electrodes. For the FBSS affecting the lumbosacral area, an electrode was placed in the T12/L1 (66%) and T8/9 (34%), respectively. When the sensory deficit in the painful area was graded according to the scale of no, mild and moderate to severe degree, about 48% of FBSS patients did not show any sensory deficit. However, the presence of moderate to severe sensory loss was found in 34% of all patients and this proportion was higher in the trial failure group (56.3%) than the trial success group (21.4%).
There were no significant differences between the two groups (success or failure in trial stimulation) in terms of age, degree of pain (preop. VAS), number of operations, and duration of pain (p>0.05, independent t-test). Univariate analysis revealed the type of electrode and presence of severe sensory loss were significantly associated with the success of trial stimulation (Table 2). However, the remaining variables, sex, type of pain, main location of pain, degree of pain duration, degree of sensory loss, and the presence of motor weakness, were not associated with the trial success of SCS for FBSS (Table 3).
FBSS is a term embracing a constellation of conditions that describes persistent or recurring low back pain, with or without sciatica following one or more spine surgeries25). A more functional definition proposes FBSS results when the outcome of lumbar spinal surgery does not meet the pre-surgical expectations of both the patient and surgeon39). The incidence of patients that will develop FBSS following lumbar spinal surgery is commonly quoted in the range of 10% to 40%18,19,25).
Treatment of FBSS is challenging because medical treatment and repeated back surgeries are often unsuccessful in providing adequate pain relief31). It is also known that the success rate is reduced if subsequent operations are performed. Nachemson's work revealed inferior results with each successive operation on the same patient. The initial success rate exceeded 50% but was reduced to 30% after the second surgery, 15% after the third, and to 5% after the fourth24). The impact of FBSS on an individual's quality of life and functional status are considerable and more disabling when compared with other common chronic conditions.
Interdisciplinary management is now considered as cornerstone of treatment of many chronic pain conditions, and its value has been assessed in FBSS23). Miller et al.23) reported that both FBSS and non-FBSS patients showed improvement with regard to pain and functional level with multidisciplinary treatment. Unfortunately, many FBSS patients will not achieve adequate analgesia and functional improvement with conservative measures alone16,20). These patients will require more invasive interventions including injection therapies (medial branch blocks, radiofrequency neurolysis, epidural injections, and percutaneous epidural adhesiolysis), implatableneuromodulatory therapies (SCS, intrathecal analgesic delivery implants), and revision surgery12). The evidence base for these interventions has grown in recent times. The efficacy of epidural adhesiolysis and SCS in particular are now accepted12).
SCS involves the placement of electrodes in the epidural space and production of an electrical current by means of a pulse generator32). The analgesic mechanism produced by SCS is believed to work by the gate control mechanism and modulation of excitatory and inhibitory neurotransmitter release in the dorsal horn22). Initially, SCS was seen as a therapy with some utility for patients with neuropathic/radicular pain who failed all other therapies36).
However, in 2004, a systematic review found only moderate evidence regarding the use of SCS in FBSS4). Since 2004, the argument for SCS efficacy has been strengthened with the completion of two RCTs comparing SCS with other treatments for FBSS12). North et al.27) randomized 60 patients and compared SCS (30 patients) vs. repeated lumbosacral surgery (30 patients) using the results reported at 6 months and at a mean of 2.9 years. A more recent prospective, randomized, controlled multicenter study of patients with FBSS (PROCESS) study recruited 100 patients with FBSS and compared SCS in combination with conventional medical management (CMM) (52 patients) versus CMM alone (48 patients) with follow-up at 6, 12, and 24 months16). The primary outcome measure in both studies was the proportion of patients who had 50% or greater pain relief. These studies indicated that there is strong evidence for the efficacy of SCS in appropriately selected patients with FBSS.
A concern with SCS has been the high cost associated with the insertion and maintenance of these devices4). According to Manca et al.4), follow-up at 6 months demonstrated a significantly greater health care cost for the SCS group (CAD 19486) vs. the CMM group (CAD 3994), the mean adjusted difference being CAD 153954). However, they pointed out that the gain in health-related quality of life was considerably greater in the SCS group [EQ-5D score difference of 0.21 at 6 months (p<0.001)]4).
A systematic review by Bala et al.3), including the topic of cost-effectiveness, suggested that when measured long term, SCS is more effective and less costly, but there is an initial high cost with the implantation and maintenance of the device. However, these cost-effectiveness studies have been criticized due to the lack of calculation of cost-effectiveness ratios, confounding factors in cohort designs, small sample sizes, and lack of adequately designed trials13). Notwithstanding the difficulties in establishing accurate cost-effectiveness comparisons, the accumulation of recent data points to SCS as an effective treatment modality for FBSS. For FBSS, the evidence for SCS efficacy is strongest for patients with predominantly radicular pain15,16,27).
Our study showed that the success rate of trial stimulation with paddle electrodes was higher than that of cyclindrical electrodes. The clinical practice of SCS differs between countries, institutions, and departments. In the USA, it seems that SCS systems are routinely implanted after a trial with percutaneous leads in USA31). At our institution, we place percutaneous, cylindrical leads for unilateral leg pain and paddle leads for bilateral leg pain and low back pain as well as leg pain in trial stimulation.
In cases in which trial stimulations were effective, the IPGs were connected to the leads used for the trial stimulation. This difference in using trial stimulation leads may explain our finding that paddle leads showed a better success rate than cylindrical leads in our study. Although the implantation of the SCS system with percutaneous, cylindrical electrodes is less invasive, there are several distinct advantages to paddle-style SCS leads31). Lead migration and positional effects are commonly observed with percutaneous leads. These effects can be minimized with paddle electrodes, which have been shown to provide more consistent coverage of painful areas with paresthesia and optimize stimulation efficacy28). Paddle electrodes have also been shown to be more clinically effective and to reduce long-term stimulation related side-effects30) compared with percutaneous leads at relatively short follow-up intervals29). In the report regarding long-term outcome of SCS with paddle electrodes by Sears et al.31), they stressed thatpatients with FBSS and CRPS treated by SCS with paddle electrodes showed a high degree of satisfaction, indexed as willingness to undergo the same procedure again for the same outcome, at a mean follow-up of approximately four years.
Presence of severe sensory deficits gave a negative influence in the results of trial stimulation. In our experience, FBSS patients who showed severe hypesthesia or anesthesia dorolosa in the painful area following lumbosacral nerve root injury did not feel any stimulation-induced paresthesia of SCS even with adequate anatomical placement of trial electrodes. Some patients with moderate hypesthesia with dysesthetic pain frequently felt stimulation-induced paresthesia as an unpleasant, annoying sensation, and most of them reported the trial SCS stimulation ineffective. Although SCS has been reported to be effective for neuropathic radicular pain from FBSS15,16,27), SCS was not effective for severe neuropathic pain with profound sensory deficits in our trial stimulation. Indeed, SCS has already been known to be rarely effective for pain following spinal cord injury or myelitis where the substrate of SCS, the dorsal column, has been damaged.
Weak efficacy of SCS is also found in reports dealing SCS for postherpetic neuralgia34,40). Cases with severe dorsal cord injury with varicella zoster virus propagation are intractable to SCS due to the formation of painful areas caused by sensory nerve disturbance40). We felt that when severe sensory deficit occurs in the painful dermatome in FBSS, most patients did not feel any stimulation paresthesia, but some felt unpleasant dysesthetic sensation from SCS. The other variables in our study, age, sex, pain duration, severity of pain, type of predominant pain, location of predominant area (axial or limb), and location of lead (T8/9 or T12/L1), did not influence the results of trial stimulation.
We recognize that psychological characteristics were not investigated in our study. Although we excluded candidates with psychopathological or substance abuse and those with significant unresolved issues of secondary gain and worker's compensation, this does not necessarily mean that psychological factors were not related to the success of trial stimulation of SCS.
Original recommendations for SCS patient selection included some psychological criteria such as emotional stability and the absence of depression14); indeed, psychological evaluation is often a mandatory part of the pre-screening process prior to consideration for implantable pain-management devices9). In general, a handful of risk factors are identified that correlate with a greater risk for unsuccessful outcomes from pain treatment, including pain chronicity and duration, psychological distress, pain-related catastrophizing, a history of abuse or trauma, nicotine use and substance abuse history, poor social support, and significant cognitive deficits38).
It is widely recognized that patients with chronic pain frequently report depression, anxiety, irritability, history of physical/sexual abuse, a personal and family history of mood disorder, and other risk factors for their deleterious pain-related outcomes2). Celestin et al.11) reported that a strong association between psychological factors and treatment outcome in 92% of review studies investigating pain related functional outcomes from lumbar surgery or SCS. Presurgical psychological factors including somatization, depression, anxiety, and poor coping were most predictive of poor response to both lumbar surgery and SCS. They found that older age and longer pain duration were also predictive of poor outcome, while pre-treatment physical findings, activity interference, and pain intensity were minimally predictive.
FBSS is a chronic pain condition that has considerable impact on the patient and health care system. Management of FBSS with multiple modalities includes interventional techniques that result in moderate improvement but leaving a proportion of patients in intractable pain. SCS is an effective means of treatment of chronic neuropathic pain from FBSS. Because successful trial stimulation is an essential part of SCS, we investigated factors associated with the success of trial stimulation. In our study, the presence of severe sensory deficits and the use of paddle leads in trial stimulation were associated with the success of trial stimulation.
References
1. Allegri M, Arachi G, Barbieri M, Paulin L, Bettaglio R, Bonetti G, et al. Prospective study of the success and efficacy of spinal cord stimulation. Minerva Anestesiol. 2004; 70:117–124. PMID: 14997084.
2. Andersson HI, Ejlertsson G, Leden I, Scherstén B. Impact of chronic pain on health care seeking, self care, and medication. Results from a population-based Swedish study. J Epidemiol Community Health. 1999; 53:503–509. PMID: 10562870.

3. Bala MM, Riemsma RP, Nixon J, Kleijnen J. Systematic review of the (cost-)effectiveness of spinal cord stimulation for people with failed back surgery syndrome. Clin J Pain. 2008; 24:741–756. PMID: 18936591.

4. Beersen N, Redekop WK, de Bruijn JH, Theuvenet PJ, Berg M, Klazinga NS. Quality based social insurance coverage and payment of the application of a high cost medical therapy : the case of spinal cord stimulation for chronic non-oncologic pain in The Netherlands. Health Policy. 2005; 71:107–115. PMID: 15563997.

5. Bouhassira D, Attal N, Alchaar H, Boureau F, Brochet B, Bruxelle J, et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain. 2005; 114:29–36. PMID: 15733628.

6. Broggi G, Servello D, Dones I, Carbone G. Italian multicentric study on pain treatment with epidural spinal cord stimulation. Stereotact Funct Neurosurg. 1994; 62:273–278. PMID: 7631081.

7. Burchiel KJ, Anderson VC, Wilson BJ, Denison DB, Olson KA, Shatin D. Prognostic factors of spinal cord stimulation for chronic back and leg pain. Neurosurgery. 1995; 36:1101–1110. discussion 1110-1111. PMID: 7643988.

8. Burton CV. Failed back surgery patients : the alarm bells are ringing. Surg Neurol. 2006; 65:5–6. PMID: 16378838.
9. Campbell CM, Jamison RN, Edwards RR. Psychological screening/phenotyping as predictors for spinal cord stimulation. Curr Pain Headache Rep. 2013; 17:307. PMID: 23247806.

10. Canlas B, Drake T, Gabriel E. A severe case of complex regional pain syndrome I (reflex sympathetic dystrophy) managed with spinal cord stimulation. Pain Pract. 2010; 10:78–83. PMID: 19863748.

11. Celestin J, Edwards RR, Jamison RN. Pretreatment psychosocial variables as predictors of outcomes following lumbar surgery and spinal cord stimulation : a systematic review and literature synthesis. Pain Med. 2009; 10:639–653. PMID: 19638142.

13. Chou R. Generating evidence on spinal cord stimulation for failed back surgery syndrome : not yet fully charged. Clin J Pain. 2008; 24:757–758. PMID: 18936592.

14. Doleys DM. Psychological factors in spinal cord stimulation therapy : brief review and discussion. Neurosurg Focus. 2006; 21:E1. PMID: 17341042.
15. Frey ME, Manchikanti L, Benyamin RM, Schultz DM, Smith HS, Cohen SP. Spinal cord stimulation for patients with failed back surgery syndrome : a systematic review. Pain Physician. 2009; 12:379–397. PMID: 19305486.
16. Kumar K, Taylor RS, Jacques L, Eldabe S, Meglio M, Molet J, et al. Spinal cord stimulation versus conventional medical management for neuropathic pain : a multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain. 2007; 132:179–188. PMID: 17845835.

17. Kumar K, Taylor RS, Jacques L, Eldabe S, Meglio M, Molet J, et al. The effects of spinal cord stimulation in neuropathic pain are sustained : a 24-month follow-up of the prospective randomized controlled multicenter trial of the effectiveness of spinal cord stimulation. Neurosurgery. 2008; 63:762–770. discussion 770. PMID: 18981888.

18. Law JD, Lehman RA, Kirsch WM. Reoperation after lumbar intervertebral disc surgery. J Neurosurg. 1978; 48:259–263. PMID: 146731.

19. Lehmann TR, LaRocca HS. Repeat lumbar surgery. A review of patients with failure from previous lumbar surgery treated by spinal canal exploration and lumbar spinal fusion. Spine (Phila Pa 1976). 1981; 6:615–619. PMID: 6461073.
20. Manca A, Kumar K, Taylor RS, Jacques L, Eldabe S, Meglio M, et al. Quality of life, resource consumption and costs of spinal cord stimulation versus conventional medical management in neuropathic pain patients with failed back surgery syndrome (PROCESS trial). Eur J Pain. 2008; 12:1047–1058. PMID: 18359255.

21. Meglio M, Cioni B, Visocchi M, Tancredi A, Pentimalli L. Spinal cord stimulation in low back and leg pain. Stereotact Funct Neurosurg. 1994; 62:263–266. PMID: 7631079.

22. Meyerson BA, Linderoth B. Mode of action of spinal cord stimulation in neuropathic pain. J Pain Symptom Manage. 2006; 31(4 Suppl):S6–S12. PMID: 16647596.

23. Miller B, Gatchel RJ, Lou L, Stowell A, Robinson R, Polatin PB. Interdisciplinary treatment of failed back surgery syndrome (FBSS) : a comparison of FBSS and non-FBSS patients. Pain Pract. 2005; 5:190–202. PMID: 17147581.

24. Nachemson AL. Evaluation of results in lumbar spine surgery. Acta Orthop Scand Suppl. 1993; 251:130–133. PMID: 8451971.

25. North RB, Campbell JN, James CS, Conover-Walker MK, Wang H, Piantadosi S, et al. Failed back surgery syndrome : 5-year follow-up in 102 patients undergoing repeated operation. Neurosurgery. 1991; 28:685–690. discussion 690-691. PMID: 1831546.
26. North RB, Ewend MG, Lawton MT, Piantadosi S. Spinal cord stimulation for chronic, intractable pain : superiority of "multi-channel" devices. Pain. 1991; 44:119–130. PMID: 2052378.

27. North RB, Kidd DH, Farrokhi F, Piantadosi SA. Spinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain : a randomized, controlled trial. Neurosurgery. 2005; 56:98–106. discussion 106-107. PMID: 15617591.
28. North RB, Kidd DH, Olin JC, Sieracki JM. Spinal cord stimulation electrode design : prospective, randomized, controlled trial comparing percutaneous and laminectomy electrodes-part I : technical outcomes. Neurosurgery. 2002; 51:381–389. discussion 389-390. PMID: 12182776.

29. North RB, Kidd DH, Petrucci L, Dorsi MJ. Spinal cord stimulation electrode design : a prospective, randomized, controlled trial comparing percutaneous with laminectomy electrodes : part II-clinical outcomes. Neurosurgery. 2005; 57:990–996. discussion 990-996. PMID: 16284568.

30. North RB, Lanning A, Hessels R, Cutchis PN. Spinal cord stimulation with percutaneous and plate electrodes : side effects and quantitative comparisons. Neurosurg Focus. 1997; 2:e3. PMID: 15096024.
31. Sears NC, Machado AG, Nagel SJ, Deogaonkar M, Stanton-Hicks M, Rezai AR, et al. Long-term outcomes of spinal cord stimulation with paddle leads in the treatment of complex regional pain syndrome and failed back surgery syndrome. Neuromodulation. 2011; 14:312–318. discussion 318. PMID: 21992424.

32. Shealy CN, Mortimer JT, Reswick JB. Electrical inhibition of pain by stimulation of the dorsal columns : preliminary clinical report. Anesth Analg. 1967; 46:489–491. PMID: 4952225.
33. Sparkes E, Raphael JH, Duarte RV, LeMarchand K, Jackson C, Ashford RL. A systematic literature review of psychological characteristics as determinants of outcome for spinal cord stimulation therapy. Pain. 2010; 150:284–289. PMID: 20603026.

34. Spiegelmann R, Friedman WA. Spinal cord stimulation : a contemporary series. Neurosurgery. 1991; 28:65–70. discussion 70-71. PMID: 1704492.
35. Taylor RS. Spinal cord stimulation in complex regional pain syndrome and refractory neuropathic back and leg pain/failed back surgery syndrome : results of a systematic review and meta-analysis. J Pain Symptom Manage. 2006; 31(4 Suppl):S13–S19. PMID: 16647590.
36. Taylor RS, Van Buyten JP, Buchser E. Spinal cord stimulation for chronic back and leg pain and failed back surgery syndrome : a systematic review and analysis of prognostic factors. Spine (Phila Pa 1976). 2005; 30:152–160. PMID: 15626996.

37. Taylor RS, Van Buyten JP, Buchser E. Spinal cord stimulation for complex regional pain syndrome : a systematic review of the clinical and cost-effectiveness literature and assessment of prognostic factors. Eur J Pain. 2006; 10:91–101. PMID: 16310712.
38. Tunks ER, Crook J, Weir R. Epidemiology of chronic pain with psychological comorbidity : prevalence, risk, course, and prognosis. Can J Psychiatry. 2008; 53:224–234. PMID: 18478825.

39. Waguespack A, Schofferman J, Slosar P, Reynolds J. Etiology of long-term failures of lumbar spine surgery. Pain Med. 2002; 3:18–22. PMID: 15102214.

40. Yanamoto F, Murakawa K. The effects of temporary spinal cord stimulation (or spinal nerve root stimulation) on the management of early postherpetic neuralgia from one to six months of its onset. Neuromodulation. 2012; 15:151–154. discussion 154. PMID: 22376181.

Fig. 1
Location and types of electrodes according to pain location. A : A cylindrical lead placed at T12/L1 level for unilateral limb pain. B : Two cylindrical leads at T12/L1 level for bilateral limb pain. C : A paddle lead (2 column array) at T12/L1 for bilateral limb pain. D : A paddle lead (3 column array) at T8/9 for complex pain (low back and limbs).

Table 1
Demographic datas of patients with chronic pain from failed back surgery syndrome who underwent trial of spinal cord stimulation





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download