INTRODUCTION
Spinal subarachnoid hemorrhage (SAH) is a rare event and accounts for less than 1% of all cases reported in the literatures
1). The most common etiology of spinal SAH is bleeding from a spinal cord arteriovenous malformation (AVM) and arteriovenous fistula (AVF), which can lead to formation of an aneurysm in a feeding artery. Also, spinal SAH is usually associated with hemorrhage from intraspinal neoplasms (e.g., neurinoma, ependymoma, and hemangioblastoma), polyarteritis nodosa, syphilis, aortic coarctation, infection, connective tissue disease, and trauma
3). Solitary aneurysms of spinal arteries lacking associated above mentioned entities are extremely rare
8). Only 6 cases of ruptured solitary aneurysm of Adamkiewicz artery have been reported.
We present a case of a ruptured dissecting aneurysm of the radiculomedullary branch of the Adamkiewicz artery associated with SAH.
Go to :

CASE REPORT
A 45-year-old female patient was referred to our emergency department with a sudden burst headache and severe back pain, followed by nausea. On admission, she was alert, oriented, and cooperative. On neurological examination, she had moderate neck stiffness with mild lower limb motor weakness of IV/V. She had no predisposing clinical disease history and trauma history. Laboratory examinations showed no abnormal findings suggestive of inflammation or infection.
No abnormal findings were observed on brain computed tomography. However, brain magnetic resonance imaging (MRI) revealed thin SAH in right parietal lobe cortex. Whole spine MRI showed spinal SAH from T5 to sacrum with a small intradural extramedullary signal void lesion at the T12 level (
Fig. 1). Spinal angiography revealed a small pearl and string-like aneurysm of a radiculomedullary branch of the left segmental artery (Adamkiewicz artery) originating on the left side at the L1 level (
Fig. 2). Arteriovenous shunts or abnormal veins were not seen.
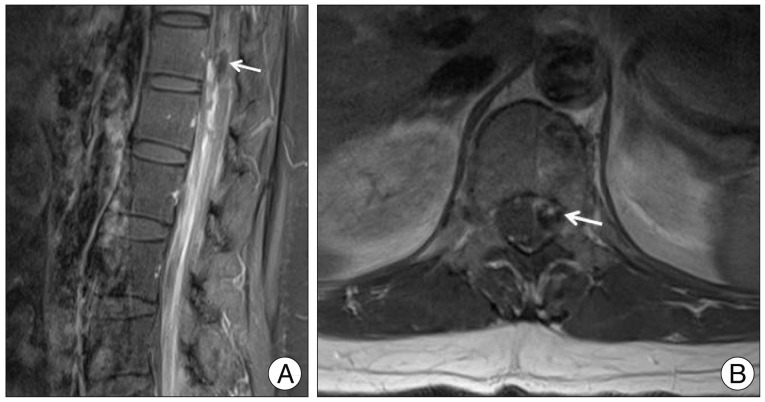 | Fig. 1Initial enhanced lumbar MRI. Sagittal image (B) view reveals thick SAH and focal intradural extramedullary mass lesion measuring 4×13 mm (white arrow) at the T12 level. Axial (A) view reveals focal mass lesion with peripheral rim enhancement (white arrow) at left anterolateral side of spinal cord. SAH : spinal subarachnoid hemorrhage. 
|
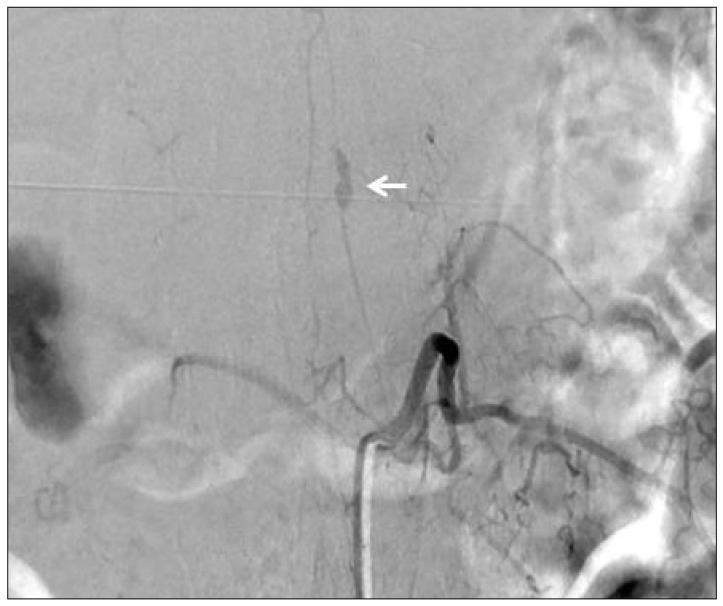 | Fig. 2Spinal angiography of the segmental artery at the left L1 level shows fusiform aneurysm of the radiculomedullary branch of the Adamkiewicz artery with a diameter of approximately 3×11 mm. 
|
The findings and treatment options were discussed with the patient's family, and we refrained from performing operative clipping or endovascular coiling of the aneurysm because of the high risk of occlusion of the anterior spinal artery during the procedure.
Over time, the patient improved gradually and was discharged without new neurological deficits.
However, one month after onset, she complained aggravation of back pain and radiating pain of both legs, and follow-up lumbar MRI showed decreased hematoma and developed arachnoiditis of cauda equina (
Fig. 3).
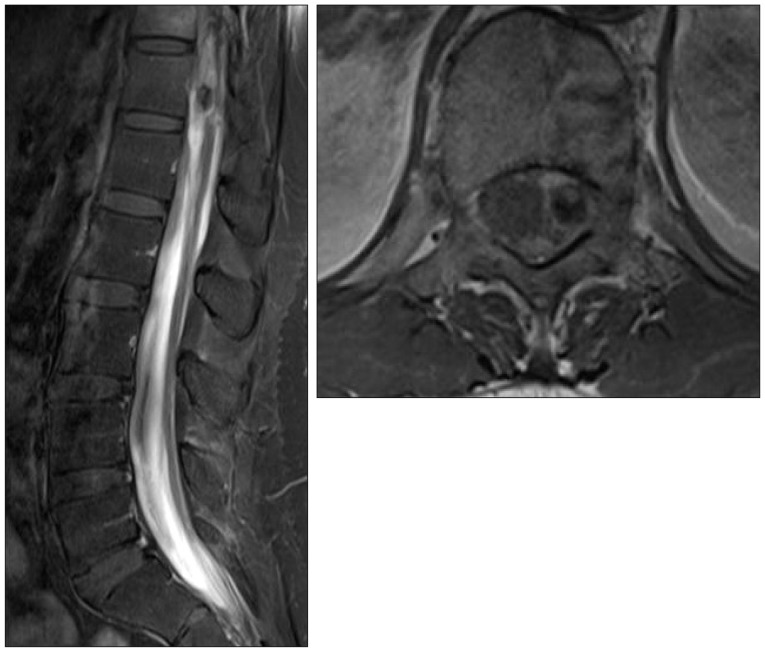 | Fig. 3Follow-up enhanced lumbar MRI at one month after onset. Sagittal and axial views revealing slightly decreased hematoma with developed arachnoiditis of cauda equina and mass lesion at the T12 level. 
|
Two months after the initial onset, her symptoms improved completely, and follow-up lumbar MRI showed resolution of SAH with septated intradural fluid collection and remaining signal void lesion at the T12 level. The patient's subsequent clinical follow-up of five months was uneventful (
Fig. 4).
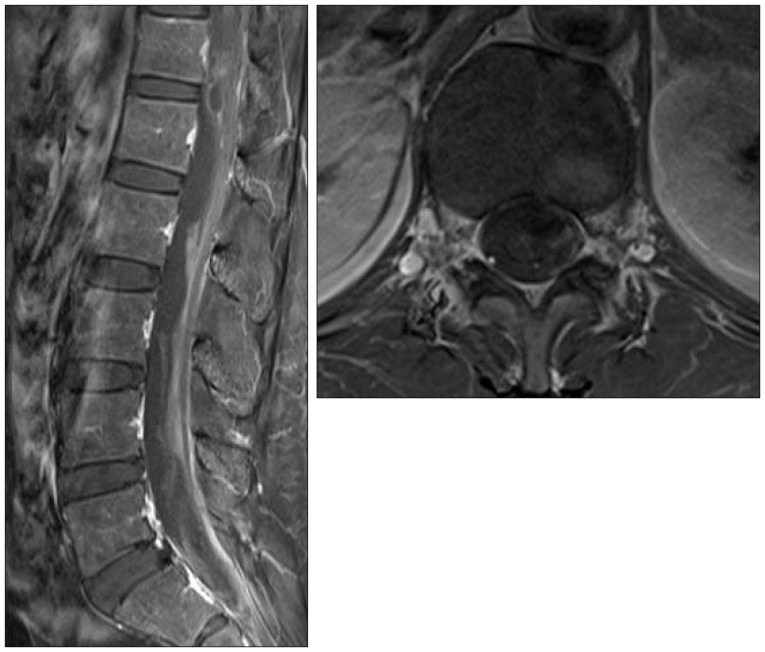 | Fig. 4Follow-up enhanced lumbar MRI at two months after onset. Sagittal and axial views showing resolution of SAH with septated intradural fluid collection and remaining mass lesion at the T12 level. 
|
Go to :

DISCUSSION
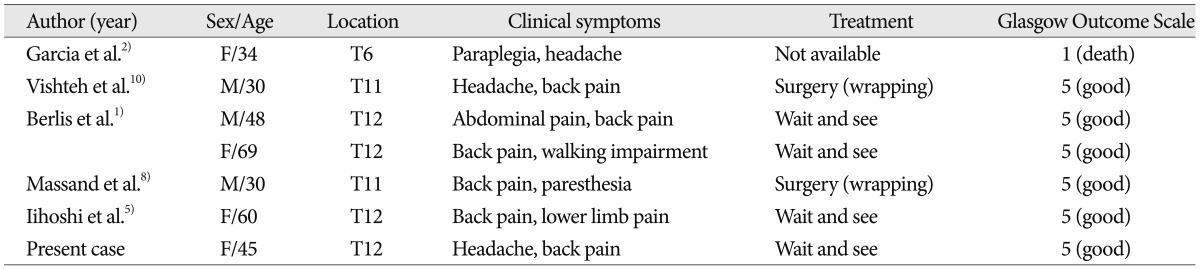
Solitary aneurysms of spinal arteries are rare, and especially, solitary aneurysms of Adamkiewicz artery are extremely rare. Only 7 cases of ruptured solitary aneurysm of Adamkiewicz artery, including the present case, have been reported (
Table 1)
1,
2,
5,
8,
10).
Table 1
Reported cases of ruptured solitary spinal artery aneurysms of the Adamkiewicz artery

Several pathogenesis of solitary spinal artery aneurysms, including inflammatory processes, arterial dissections, and intimal defects, have been proposed as potential etiologies for these rare isolated lesions
4-
6,
9). Although, the exact etiology of this aneurysm remains speculative, in the present case, according to morphology of fusiform aneurysm, we hypothesized that dissection, as primary event, resulted in the formation of a pseudoaneurysm with subsequent hemorrhage. This aneurysm could have been primarily classified as pseudoaneurysm or dissecting aneurysm.
Spinal aneurysms differ from intracranial aneurysms in several ways. In most cases, intracranial aneurysms that rupture are saccular or berry aneurysms occurring at bifurcation points along the intracranial arterial tree. Otherwise, most spinal artery aneurysms are small with diameter of <3 mm, and although both saccular and fusiform aneurysms have been identified, the latter are more frequent.
Fusiform aneurysms indicate dissecting dilatations of spinal artery and usually lack a clear neck. In addition, solitary spinal aneurysms tend to occur along the intradural portion of the radicular artery as it courses to their spinal cord rather than branch point. The majority of spinal aneurysms involve either the artery of Admakiewicz or the thoracolumbar radicular branches. The anterior spinal artery is often involved, but aneurysms can involve the posterior spinal artery or lateral spinal artery
3). The caliber of the spinal arteries is much smaller than that of intracranial arteries, and they tend to be less affected by atherosclerosis
8).
Partial thrombosis or acute dissection of spinal aneurysms is a common surgical finding and probably accounts for their symptoms. Spinal aneurysms can manifest as compressive lesions, but they typically present with rupture. The specific symptoms of a ruptured spinal aneurysm seem to correlate well with the level and severity of hemorrhage; however, the abrupt onset of pain appears universal
3). Lesions involving the upper cervical spine usually become symptomatic with intracranial SAH or quadriparesis
4,
9). Lesions in the thoracolumbar spine can cause headache, nuchal pain, abdominal pain, lower back pain, sensory disturbance, or acute paraparesis
6). Also, an unruptured aneurysm in the cervical or thoracic spine manifested with compressive myelopathy
3). Our case is unusual because of the aneurysm location at T12 and the presentation with SAH of parietal lobe causing headache and nausea.
Although spinal aneurysms are a rare cause of intracranial SAH, the diagnosis should be considered when no other source of hemorrhage can be identified or when the SAH is limited to the spine. Furthermore, when patient complains increasing intracranial pressure sign, meningeal irritation sign, and severe trunk pain simultaneously, spinal vascular disease should be considered. For focal lesions, aneurysms should be included in the differential diagnosis, and angiography should be performed. If the findings of angiography are negative, thrombosed aneurysm should still be considered. The differential diagnosis should be directed toward the exclusion of different types of spinal vascular disease (e.g., AVM or AVF), vasculitis, and noninflammatory vasculopathies before the performing of surgical or endovascular treatment.
Spinal aneurysms associated with hemodynamic flow states, such as AVM or AVF, have been successfully treated using endovascular and surgical techniques. These types of aneurysms are more amenable to treatment because they are typically associated with robust collateral flow to the anterior spinal artery
7). However, no standard therapy has been established because of the infrequence of SAH from a ruptured isolated spinal artery aneurysm. Therefore, the controversy on clipping and trapping or coil embolization or conservative treatment is not resolved.
Surgical treatment of spinal artery aneurysms may be required, depending on whether or not blood flow is present distal to the lesion, and offers the potential advantage of preservation of the nearby perforators, as well as the possibility of vascular bypass. The mass effect of aneurysms or surrounding blood clots may be the indication of surgical treatment. The anterior spinal artery and the artery of Adamkiewicz must be preserved, and the aneurysm can be resected. However, in general, because these aneurysms are fusiform and lack a surgical neck, direct clipping is usually not possible. If there is no evidence of flow distal to the aneurysm, the parent vessel can be sacrificed and the aneurysm can be resected without risk. If there is evidence of distal blood flow at surgery and the aneurysm cannot be directly clipped, the lesion can be wrapped and clip reinforced to minimize the risks of recurrence
10). When the bulk of the aneurysm makes wrapping impossible, direct microvascular reconstruction can be attempted. The position of the aneurysm with respect to the spinal cord-anterior, lateral, or posterior-is a key determinant of the surgical approach. If the aneurysm is located anterolateral or posterior to the spinal cord, a traditional posterior laminotomy can be used. Sectioning the dentate ligament improves exposure from a posterior approach and also can be used to retract the spinal cord. If the aneurysm is located in the midline, anterior to the spinal cord in the lower thoracic region, anterior approach can be used. During the operation, somatosensory and motor evoked potentials must be recorded.
Endovascular treatment may be an appropriate choice, especially if surgery is difficult considering the location of the lesion or the condition of the patient. The use of the small-size catheter may prevent undirected local occlusion and spasm of spinal artery. Endovascular procedures can be used to occlude only aneurysm or occlude the parent vessel when there is little or no flow distal to the lesion
7).
Alternative to surgical or endovascular treatment, limited to isolated spinal dissecting aneurysm with the small size of artery and the presence of distal flow, spontaneous healing of the dissection and obliteration of the aneurysm may be the possible approach. In 2005, Berlis et al.
1) proposed a wait-and-see strategy for these aneurysms. Our patient had classical fusiform aneurysm with no surgical neck and with blood flow distal to the lesion. Surgical clipping of aneurysm or trapping of parent artery carries high risk of injury of Adamkiewicz artery during the procedure. Also, endovascular treatment was not amenable due to the inaccessibility of the anterior spinal artery and risk of occlusion of Adamkiewicz artery during intervention. As a result, in our case, conservative therapy may be preferred because of the small size of the artery and the presence of distal flow. If surgical intervention is delayed for any reason or if emergency surgery is not needed, repeat angiography should be considered before initiation of any delayed intervention, because spontaneous regression is likely.
Go to :






 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download