Abstract
Objective
To examine the synergistic effects of both computer-assisted cognitive rehabilitation (CACR) and transcranial direct current stimulation (tDCS) on cognitive function in patients with stroke.
Methods
The current double-blind, sham-controlled study enrolled a total of 11 patients who were newly diagnosed with stroke. The patients of the tDCS group (n=6) completed sessions of the Korean computer-assisted cognitive rehabilitation program five times a week for 30 minutes a session during a mean period of 18.5 days concomitantly with the anodal tDCS over the bilateral prefrontal cortex combined with the CACR. The patients of the control group (n=5) also completed sessions of the sham stimulation during a mean period of 17.8 days. Anodal tDCS over bilateral prefrontal cortex (F3 and F4 in 10-20 EEG system) was delivered for 30 minutes at an intensity of 2 mA. Cathode electrodes were applied to the non-dominant arm. All the patients were evaluated using the Seoul Computerized Neuropsychological Test (SCNT) and the Korean Mini-Mental State Examination.
Cognitive dysfunction is known as one of the common complications that occur after stroke and it accounts for 10-82% of total patients with stroke24,25). Cognitive impairment after stroke can cause serious social problems, and these include decreased quality of life of patients and severe economic loss with an increase in the medical expense by the community members. It would therefore be mandatory to accurately assess and to perform rehabilitation treatments of patients who present with cognitive impairment, which is essential for achieving successful treatment outcomes in patients with stroke and helping them to return to the daily lives8).
A computer-assisted cognitive rehabilitation (CACR) has been frequently used since it was first used by Glisky et al.11) in 1986. It is used to enhance the degree of cognition of patients with brain injury. Moreover, it is advantageous in providing the standardized task and the immediate feedback for task performance. Furthermore, it is useful in performing a follow-up of patients' clinical course and conducting a clinical study by constantly analyzing and comparing data about the performance results. It has been reported that memory, auditory and visual attention were significantly improved using the Korean computer-assisted cognitive rehabilitation program in patients with brain injury14).
Recent ongoing studies examine the clinical usefulness of non-invasive cerebral cortex stimulation technique such as transcranial direct current stimulation (tDCS) in the treatment of patients with brain disorder. Andrews et al.1) reported that the working memory was improved by stimulating the dorsolateral prefrontal cortex with tDCS in healthy adults. In addition, a variety of studies have shown that the tDCS had a significant effect in rehabilitating the cognitive functions in patients with brain injury20). Jo et al.12) demonstrated that the tDCS over the left dorsolateral prefrontal cortex was associated with enhanced working memory performance. Ko et al.17) showed that the tDCS was applied to the scalp over the right posterior parietal cortex and it was effective in improving the visual scanning performance in stroke patients with spatial neglect. The tDCS is a non-invasive brain stimulation technique and there is strong evidence that neurons underlying the anode are 'excited', with resting membrane potential shifting towards depolarization and an increased rate of spontaneous neuronal firing27).
It has already been reported that the effect of either CACR or tDCS on cognitive function. But there are insufficient studies about the effect of both CACR and tDCS on cognitive function.
Given the above background, we conducted this study to examine the synergistic effects of both CACR and tDCS on cognitive function in patients with stroke. To do this, we performed cognitive function test with the Korean Mini-Mental State Examination (K-MMSE) and Seoul Computerized Neuropsychological Test (SCNT).
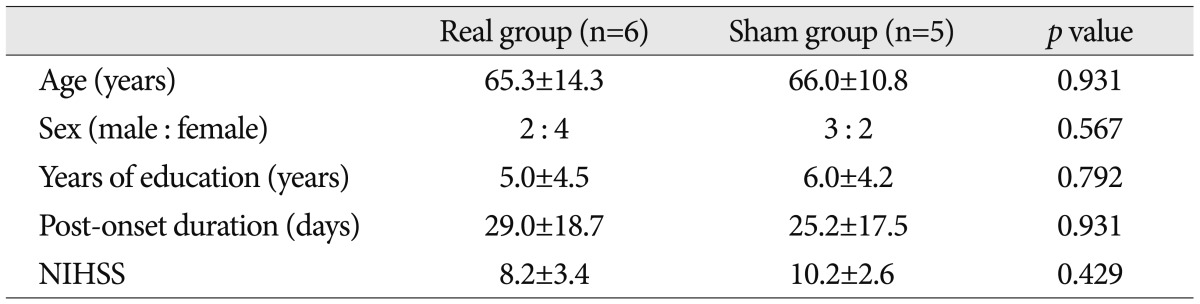
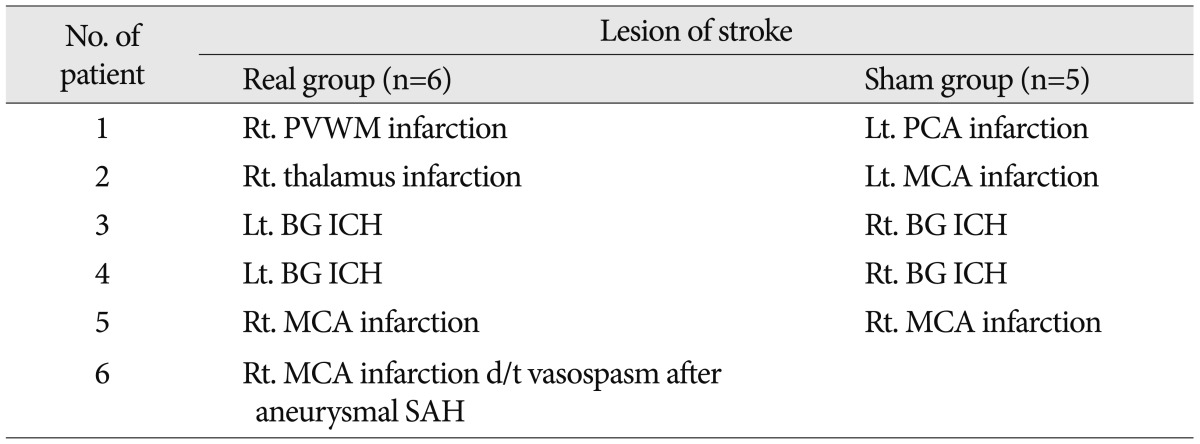
The current study enrolled a total of 11 patients, comprising five men and six women, whose mean age 65.6 years old. They were newly diagnosed with stroke, whose lesions were confirmed by a magnetic resonance imaging (MRI) or a computerized tomography. Of them, six patients had cerebral infarction, four had cerebral hemorrhage and the remaining one had secondary cerebral infarction due to vasospasm after aneurysmal subarachnoid hemorrhage. In addition, there were six right-sided cases and five left-sided one. Moreover, they had no notable history of brain diseases, including dementia, that could cause cognitive dysfunction other than stroke. To rule out significant aphasia, patients underwent bedside language screening test which was consisted of 5 language domains such as comprehension, production, reading, writing, and repetition. All patients showed normal values in the bedside language screening test.
The patients were composed of mild-to-moderate cases of cognitive dysfunction, and participated in the SCNT by pushing the button according to our instructions. Their K-MMSE scores were lower than 25 points, whose mean value was 18.36 points (range, 10-24 points) prior to the combination of tDCS with CACR. Their years of education were 5.5 years on average. The patients equipped with metal in the head and the subjects with skin lesions in electrode attachment site were excluded. The institutional review board of our hospital approved the study protocol, and written informed consent was obtained from all subjects before participation. The patients were randomly assigned to two groups : the tDCS group (n=6) and the control group (n=5). Between the two groups, however, there were no significant differences in the age, sex, years of education, the disease duration and the National Institutes of Health Stroke Scale score during the hospital stay (Table 1). The location of lesions and etiologic causes are summarized in Table 2.
As a transcranial direct current stimulator, we used the Phoresor II Auto Model PM850 (IOMED®, Salt Lake City, UT, USA) generating direct current with batteries, where the sponge electrodes of 5×5 cm in size (area : 25 cm2) were attached. The anode and the cathode were attached to the bilateral prefrontal cortex and the non-dominant arm, respectively. Then, they were entirely attached to the sites. The real stimulus was set at an intensity of 2 mA for 30 minutes. Meanwhile, the Korean computer-assisted cognitive rehabilitation program was concomitantly applied. The sham stimulus was set identical except that the current was reduced to zero after 30 seconds in such a manner that the patients were blinded to the turning-off of the power. The tDCS and the cognitive function test were performed by two independent personnels.
The Korean computer-assisted cognitive rehabilitation program (ComCog®, v1.0, Maxmedica, Seoul, Korea, 2004) consists of ten traning programs related to the attention and another ten related to the memory. In combination with the tDCS, the patients underwent the cognitive rehabilitation program 30 minutes (a 15-minute attention training and a 15-minute memory training) a day five times a week until the discharge since the hospitalization at the rehabilitation center. The cognitive rehabilitation program and the tDCS were performed by the same personnel.
Cognitive function test was performed by implementing the K-MMSE and the SCNT (SCNT®, v2.0, Maxmedica, Seoul, Korea, 2001) prior to and following the concomitant use of tDCS with CACR.
The SCNT were composed of a total of ten items and it was performed by a single personnel. These ten items include digit span test, verbal learning test for verbal memory function, visual span test, visual learning test for visuospatial memory, auditory continuous performance test (CPT), auditory controlled CPT, visual CPT, visual controlled CPT, word-color test for attention function, and trail making test for visuomotor coordination function15).
The post/pre ratio of scores on the K-MMSE and SCNT item was calculated between prior to and following the concomitant use of tDCS with CACR.
The post/pre ratio of scores on the K-MMSE and SCNT item was compared using the Mann-Whitney U test between the two groups. Statistical analysis was done using the Statistical Package for Social Science (SPSS) 18.0 (SPSS Inc., Chicago, IL, USA). All the data were expressed as mean±standard deviation. Statistical significance was set at p<0.05.
Some patients had pricking sensation at the sites of stimulation after the tDCS but there were no notable side effects. The mean period of CACR was 18.5 days in the tDCS group and 17.8 days in the control group.
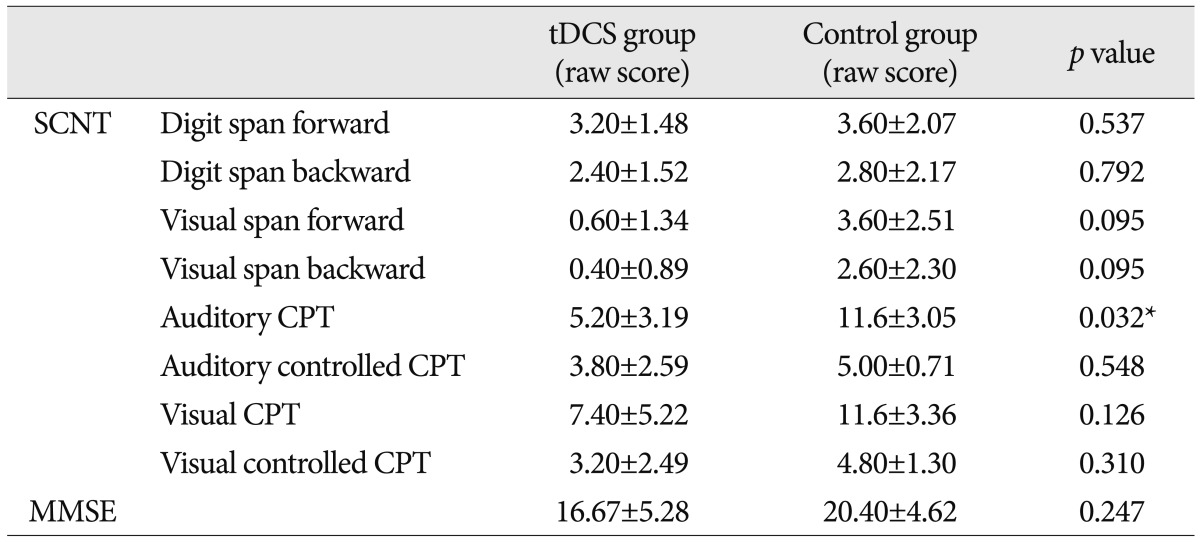
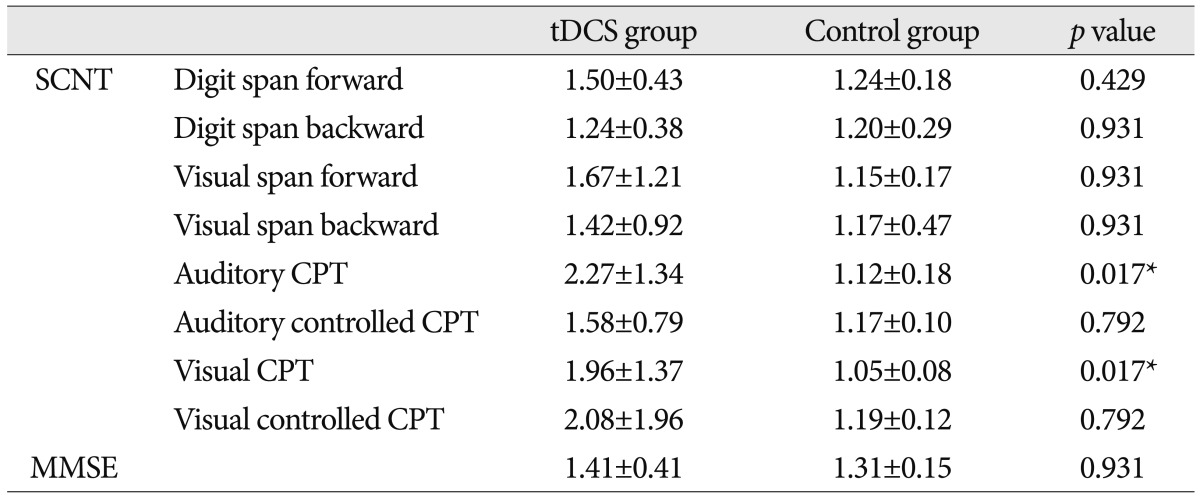
There were no significant differences in the SCNT scores between the two groups at baseline except auditory CPT (p=0.032) (Table 3). The post/pre ratio of scores on the SCNT item was significantly higher in the tDCS group as compared with the control group on the auditory CPT (p=0.017) and visual CPT (p=0.017) for attention function (Table 4).
Of note, the simultaneous application of tDCS during the CACR was more effective than the single use of CACR in improving the cognitive function after stroke. The current intensity of recent conventional tDCS is relatively very weak, therefore the tDCS does not induce the neuronal firing of resting cell. Rather, it is believed to modulate the increased rate of spontaneous neuronal firing depending on the polarity27). According to these technical characteristics, the combination of tDCS with functional training can be a more effective therapeutic approach than the single use of either tDCS or functional training.
In the current experimental study, we attached the electrodes to the prefrontal cortex. In the tDCS group, of the SCNT items, only those associated with attention had a significant improvement.
Many previous reports have also shown that the prefrontal cortex is involved in the detection of external changes and the interaction with the environment16). It has also been reported that patients with prefrontal cortex damage present with apathy16). Yamasaki et al.28) demonstrated a correlation between the attention and prefrontal cortex on functional MRI scans. Furthermore, Bloch et al.3) reported that there was an improvement in the attention following the application of repetitive transcranial magnetic stimulation to the prefrontal cortex using the non-invasive brain stimulation technique in children with attention deficit hyperactivity disorder.
In the current study, of the SCNT items, auditory CPT and visual CPT involved in the attention had a significant improvement in the tDCS group as compared with the control group. This suggests that the degree of attention can be significantly improved with the application of the tDCS to the prefrontal cortex in patients with stroke.
However, there were no significant differences in the post/pre ratio on the K-MMSE score and other SCNT items (memory function and visual motor coordination function).
The MMSE is widely used to assess the cognitive function after stroke; its Korean version, K-MMSE, is valid for testing patients with dementia7,13). And, many previous studies reported that the prefrontal cortex played a crucial role in regulating the cognitive function19). Prefrontal cortex performs short-term storage of input data, and it is also known to play a role in controlling the working memory that is closely associated with the cognitive processes such as long-term memory, language learning and executive function2,5,10). Cerebral cortexes involved in the working memory include bilateral medial posterior parietal cortex, bilateral premotor cortex, cingulate gyrus, bilateral frontal pole, bilateral dorsolateral prefrontal cortex and bilateral ventrolateral prefrontal cortex22). Previous studies have been conducted in healthy adults and Parkinsonian patients, thus reporting that there was a significant improvement in the working memory following the tDCS for prefrontal cortex9,12,21). Boggio et al.4) reported that 18 Parkinsonian patients achieved a significant improvement in the accuracy of verbal three-back working memory after receiving the tDCS to left prefrontal cortex at an intensity of 2 mA for 20 minutes. Moreover, Jo et al.12) also reported that ten patients with cerebral infarction achieved a significant improvement in the accuracy of verbal two-back working memory after receiving the tDCS to left prefrontal cortex at an intensity of 2 mA for 30 minutes. Furthermore, Fregni et al.9) also reported that 15 healthy young adults achieved a significant improvement in the accuracy of verbal three-back working memory after receiving the tDCS to left prefrontal cortex at an intensity of 1 mA for 10 minutes.
Our study did not show significant improvement in the K-MMSE scores and memory function test and visuomotor coordination function test of SCNT in the tDCS group as compared with the control group. Due to a small number of enrolled patients (n=11), the uneven distribution location and size of the lesions and the etiologic causes between the two groups is the limitations of our study. According to Desmond et al.7), the incidence of cognitive dysfunction after stroke was reduced by 54% at a long-term follow-up in patients with left-sided lesions and those with major hemispheric syndrome. These authors also noted, however, that the incidence of diabetic complications was reduced by 11.9% at a long-term follow-up. Patel et al.23) reported that the cognitive dysfunction was affected by the smoking, unilateral neglect syndrome, location of lesions and the degree of functional perfomance. In addition, the cognitive dysfunction after stroke was affected by many variables. In the current study, however, we could not match between the two groups becauce of a small number of enrolled patients. We hope further large-scale and case-control studies could show significant improvement in K-MMSE score and other SCNT items following concomitant use of tDCS with CACR.
In the current study, we failed to clarify whether there is a persistent presence of the improvement in the cognitive function following the concomitant use of the tDCS with CACR at a long-term follow-up because the cognitive function was evaluated only twice in the tDCS group (at the time of hospitalization and discharge). According to Desmond et al.7), the degree of cognitive function was improved by 35.9% at a 1-year follow-up in patients with stroke. Kotila et al.18) conducted a study in 154 patients, thus reporting that the degree of cognitive function was improved until a year since the onset of stroke. These authors also noted, however, that most of the patients achieved an improvement in the degree of cognitive function between the onset of stroke and three months after it. Wade et al.26) conducted a study in 85 patients with stroke, thus reporting that both the degree of tapping performance and that of non-verbal memory were improved between three and six months after the onset of stroke. Further long-term follow-up studies are therefore warranted to suggest the appropriate period and guidelines for the initiation and termination of the tDCS for the rehabilitation of cognitive function in patients with stroke.
Also, all patients who participated in this study had never received objective test for screening their cognitive function before stroke. Therefore, we had to check patients' previous cognitive function by history taking. If some patients would have mild cognitive impairment, themselves and their family can't notice the cognitive impairment. Although we excluded patients had history of brain diseases, including dementia, we can't convince patients' cognitive impairment absolutely induced by stroke.
Our results showed that there were no significant differences in the word-color test and trail making test scores on the SCNT items between the two groups. That is, there were no significant improvements in the items of higher difficulty. This indicates that the concomitant use of the tDCS with CACR was less effective in helping the patients to return to the daily lives.
Despite the limitations of the current study, we found that the concomitant use of non-invasive tDCS with CACR had a significant effect in improving attention in stroke patients with mild-to-moderate cognitive dysfunction. But, further studies are warranted to examine the long-term effects of the concomitant use of non-invasive tDCS with CACR on the cognitive function including memory and higher cognitive function in patients with stroke.
With the concomitant use of the tDCS with CACR, a relatively safe, convenient, non-invasive treatment modality, there was a significant improvement in the attention in the tDCS group as compared with the control group. However, this deserves further large-scale and case-control studies.
In conclusion, our results indicate that the concomitant use of the tDCS with CACR to the prefrontal cortex may provide additional beneficial effects in improving the cognitive dysfunction for patients with stroke.
References
1. Andrews SC, Hoy KE, Enticott PG, Daskalakis ZJ, Fitzgerald PB. Improving working memory : the effect of combining cognitive activity and anodal transcranial direct current stimulation to the left dorsolateral prefrontal cortex. Brain Stimul. 2011; 4:84–89. PMID: 21511208.

3. Bloch Y, Harel EV, Aviram S, Govezensky J, Ratzoni G, Levkovitz Y. Positive effects of repetitive transcranial magnetic stimulation on attention in ADHD Subjects : a randomized controlled pilot study. World J Biol Psychiatry. 2010; 11:755–758. PMID: 20521875.

4. Boggio PS, Ferrucci R, Rigonatti SP, Covre P, Nitsche M, Pascual-Leone A. Effects of transcranial direct current stimulation on working memory in patients with Parkinson's disease. J Neurol Sci. 2006; 249:31–38. PMID: 16843494.

5. Constantinidis C, Procyk E. The primate working memory networks. Cogn Affect Behav Neurosci. 2004; 4:444–465. PMID: 15849890.

6. Daffner KR, Mesulam MM, Scinto LF, Acar D, Calvo V, Faust R. The central role of the prefrontal cortex in directing attention to novel events. Brain. 2000; 123(Pt 5):927–939. PMID: 10775538.

7. Desmond DW, Moroney JT, Sano M, Stern Y. Recovery of cognitive function after stroke. Stroke. 1996; 27:1798–1803. PMID: 8841333.

8. Diamond PT, Felsenthal G, Macciocchi SN, Butler DH, Lally-Cassady D. Effect of cognitive impairment on rehabilitation outcome. Am J Phys Med Rehabil. 1996; 75:40–43. PMID: 8645438.
9. Fregni F, Boggio PS, Nitsche M, Bermpohl F, Antal A, Feredoes E. Anodal transcranial direct current stimulation of prefrontal cortex enhances working memory. Exp Brain Res. 2005; 166:23–30. PMID: 15999258.

10. Gazzaley A, Rissman J, D'Esposito M. Functional connectivity during working memory maintenance. Cogn Affect Behav Neurosci. 2004; 4:580–599. PMID: 15849899.

11. Glisky EL, Schacter DL, Tulving E. Computer learning by memory-impaired patients : acquisition and retention of complex knowledge. Neuropsychologia. 1986; 24:313–328. PMID: 3755511.

12. Jo JM, Kim YH, Ko MH, Ohn SH, Joen B, Lee KH. Enhancing the working memory of stroke patients using tDCS. Am J Phys Med Rehabil. 2009; 88:404–409. PMID: 19620953.

13. Kang Y, Na DL, Hahn S. A validity study on the Korean Mini-Mental State Examination (K-MMSE) in dementia patients. J Korean Neurol Assoc. 1997; 15:300–308.
14. Kim YH, Ko MH, Seo JH, Park SH, Kim KS, Jang EH, et al. Effect of computer-assisted cognitive rehabilitation program for attention training in brain injury. J Korean Acad Rehabil Med. 2003; 27:830–839.
15. Kim YH, Shin SH, Park SH, Ko MH. Cognitive Assessment for Patient with Brain Injury by Computerized Neuropsychological Test. J Korean Acad Rehabil Med. 2001; 25:209–216.
16. Knight RT, Grabowecky MF, Scabini D. Role of human prefrontal cortex in attention control. Adv Neurol. 1995; 66:21–34. discussion 34-36. PMID: 7771302.
17. Ko MH, Han SH, Park SH, Seo JH, Kim YH. Improvement of visual scanning after DC brain polarization of parietal cortex in stroke patients with spatial neglect. Neurosci Lett. 2008; 448:171–174. PMID: 18952147.

18. Kotila M, Waltimo O, Niemi ML, Laaksonen R, Lempinen M. The profile of recovery from stroke and factors influencing outcome. Stroke. 1984; 15:1039–1044. PMID: 6506115.

19. Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001; 24:167–202. PMID: 11283309.

20. Miniussi C, Cappa SF, Cohen LG, Floel A, Fregni F, Nitsche MA, et al. Efficacy of repetitive transcranial magnetic stimulation/transcranial direct current stimulation in cognitive neurorehabilitation. Brain Stimul. 2008; 1:326–336. PMID: 20633391.

21. Ohn SH, Park CI, Lee BH, Kim YH. Effect of prefrontal repetitive transcranial magnetic stimulationon the enhancement of working memory. J Korean Acad Rehabil Med. 2008; 32:501–505.
22. Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm : a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005; 25:46–59. PMID: 15846822.

23. Patel M, Coshall C, Rudd AG, Wolfe CD. Natural history of cognitive impairment after stroke and factors associated with its recovery. Clin Rehabil. 2003; 17:158–166. PMID: 12625656.

24. Pohjasvaara T, Erkinjuntti T, Ylikoski R, Hietanen M, Vataja R, Kaste M. Clinical determinants of poststroke dementia. Stroke. 1998; 29:75–81. PMID: 9445332.

25. Rasquin SM, Lodder J, Ponds RW, Winkens I, Jolles J, Verhey FR. Cognitive functioning after stroke : a one-year follow-up study. Dement Geriatr Cogn Disord. 2004; 18:138–144. PMID: 15211068.

26. Wade DT, Parker V, Langton Hewer R. Memory disturbance after stroke : frequency and associated losses. Int Rehabil Med. 1986; 8:60–64. PMID: 3804598.
27. Wassermann EM, Grafman J. Recharging cognition with DC brain polarization. Trends Cogn Sci. 2005; 9:503–505. PMID: 16182596.

28. Yamasaki H, LaBar KS, McCarthy G. Dissociable prefrontal brain systems for attention and emotion. Proc Natl Acad Sci U S A. 2002; 99:11447–11451. PMID: 12177452.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download