Abstract
Objective
Although curcumin has a protective effect on bone remodeling, appropriate therapeutic concentrations of curcumin are not well known as therapeutic drugs for osteoporosis. The purpose of this study was to compare the bone sparing effect of treatment of low-dose and high-dose curcumin after ovariectomy in rats.
Methods
Forty female Sprague-Dawley rats underwent either a sham operation (the sham group) or bilateral ovariectomy (OVX). The ovariectomized animals were randomly distributed among three groups; untreated OVX group, low-dose (10 mg/kg) curcumin administered group, and high-dose (50 mg/kg) curcumin group. At 4 and 8 weeks after surgery, serum biochemical markers of bone turnover were analyzed. Bone histomorphometric parameters of the 4th lumbar vertebrae were determined by micro-computed tomography (CT). In addition, mechanical strength was determined by a three-point bending test.
Results
High-dose curcumin group showed significantly lower osteocalcin, alkaline phosphatase, and the telopeptide fragment of type I collagen C-terminus concentration at 4 and 8 weeks compared with the untreated OVX group as well as low-dose curcumin group. In the analyses of micro-CT scans of 4th lumbar vertebrae, the high-dose curcumin treated group showed a significant increase in bone mineral densities (p=0.028) and cortical bone mineral densities (p=0.036) compared with the low-dose curcumin treated group. Only high-dose curcumin treated group had a significant increase of mechanical strength compared with the untreated OVX group (p=0.015).
Osteoporosis is a chronic disease of the skeleton characterized by loss of bone mass and disruption of bone microarchitecture, increasing the risk of fracture11,18). Nowadays, the incidence of osteoporosis is increasing rapidly in the elderly population, especially in postmenopausal women16,17,20).
Bisphosphonates (BPs) are potent antiresorptive agents widely used as the mainstay of treatment of osteoporosis. Because BPs accumulate in bone and are released for months or years after treatment is stopped, physicians need to be aware of the potential adverse effects of long-term use of BPs in osteoporosis3,4,7,8,21). 461Due to the possible undesirable effects associated with pharmacological treatments, natural alternatives for the prevention and treatment of osteoporosis are highly desirable1,3,6,23).
Curcumin is found in the rhizomes of the popular Indian spice turmeric plant (Curcumin longa L.), a member of the ginger family. It is well known that curcumin has diverse biologic effects, including anti-inflammatory, antioxidant, antiviral and anti-infectious5,13,19). In addition, some studies investigated the effects of curcumin on the regulation of bone remodeling. It has known as a therapeutic oriental plant that have shown to improve bone quality9). In a recent study by French et al.6), the long-term effects of curcumin administration in ovariectomized rats were examined, and it was concluded that curcumin produced beneficial changes in bone turnover and an increase in bone strength in the ovariectomized mature rat model of postmenopausal osteoporosis.
Curcumin has been consumed as a dietary spice at doses of up to approximately 1.5 mg/kg/day19). However, according to the recent study of Folwarczna et al.5), curcumin at a dietary achievable dose may not be useful for the prevention or treatment of osteoporosis.
Although curcumin has a protective effect on bone remodeling2,5,6,9,13,19,22), appropriate therapeutic concentrations of curcumin are not well known as therapeutic drugs for osteoporosis.
Thus the aim of the present study was to compare the bone sparing effect of treatment of low-dose and high-dose curcumin after ovariectomy in rats. We studied normal female rats and rats with estrogen deficiency (bilaterally ovariectomized) as a model of postmenopausal osteoporosis.
All experiments involving animals were performed in accordance with the animal care guidelines issued by the National Institutes of Health, and were approved by the Institutional Animal Care Committee at our institute.
Forty female Sprague-Dawley rats (11 weeks old) were purchased from Samtako Bio Inc. (Osan, Korea) and acclimated to conditions for one week before the experiment. Animals were housed in an air-conditioned room (relative humidity 45-65%) under a 12-h light/dark cycle at 22±2℃ and given free access to food and tap water. Animals were fed phytoestrogen reduced food (Harlan Teklad, Madison, WI, USA) and were housed in tall cages in order to provide a level of physical activity and mechanical stress considered to be osteogenic. The following week, 30 animals underwent bilateral ovariectomy (OVX) using the double dorso-lateral approach technique described in detail by Park et al.12). The remaining 10 animals underwent sham surgery and were designated as group 1 (sham group). The 30 ovariectomized animals were randomly distributed among three groups; untreated OVX group, low-dose curcumin (10 mg/kg) administered group and high-dose curcumin (50 mg/kg) administered group.
Before and four weeks after the OVX or sham operations, blood samples were collected for measurement of biochemical markers of bone turnover. Eight weeks after OVX or sham operations, all animals were sacrificed and blood samples were collected by cardiac puncture for serum isolation.
Curcumin was administered by a stomach tube (po) daily for eight weeks, and dietary intakes were limited to 20 g d-1 in order to avoid excessive body weight gains following ovariectomy. Body weights were checked once a week throughout the eight-week experimental period.
Serum was separated by centrifugation (at 1500×g) and then stored at -80℃ until required for bone metabolic marker assays. Serum estrogen (estradiol, E2) was determined using an estradiol ELISA kit (ELISA, DRG instruments GmbH, Marburg, Germany). In addition, the 4th lumbar vertebrae were removed, fixed in a 3.7% formaldehyde in phosphate-buffered saline solution (pH 7.4) for 16 h and then stored (4℃) in 80% ethanol for bone mass measurements.
Serum osteocalcin levels were determined using osteocalcin EIA kits (Nordic Bioscience Diagnostics, Herlev, Denmark) and alkaline phosphatase (ALP) activities used QuantiChrome ALP assay kits (DALP-250, BioAssay Systems, CA, USA). These are both sensitive biochemical markers of bone formation. Serum levels of C-terminal telopeptide fragment of type I collagen C-terminus (CTX-1), which is generated by the osteoclast and is a marker of bone resorption, were determined using RatLaps ELISA kits (Nordic Bioscience Diagnostics). Serum biochemical markers were expressed as a fraction of sham operated values.
Bone histomorphometric parameters and the microarchitectural properties of 4th lumbar vertebrae were determined using a micro-CT system (eXplore Locus SP, GE Healthcare, London, Ontario, Canada) with X-ray energy settings of 80 kV and 80 µA. For bone analysis, the entire 4th lumbar vertebrae were selected as the region of interest. Image information was obtained based on the automatic domain values produced by the computer. Bone mineral densities (BMD), trabecular bone volume fractions (BV/TV, %), and cortical bone mineral densities (CrBMD) were used for the quantitative analysis, which was performed using 2.0+ ABA Microview software provided with the micro-CT system.
Mechanical spinal strength was determined by a three-point bending test. Each bone was positioned on the two lower supports of the anvil of a Universal Testing Machine (Instron 4202; Instron, Canton, MA, USA). Load was applied to the midportion of the 4th lumbar vertebrae using a crosshead speed of 1.5 mm/min for all the tests. The load versus displacement data were recorded automatically by the Instron software (INSTRON series IX Automated Materials Tester, version 8.04.00, Canton, MA, USA), which calculates the mechanical parameters from the load-displacement curves.
All statistical comparisons were made using SPSS 17.0. (SPSS Inc., Chicago, IL, USA). Data are expressed as means±standard errors. Repeated measure ANOVA was used to compare body weights in the OVX and sham groups. One-way ANOVA was used to identify significant differences between the groups, and p values of ≤0.05 were considered significant.
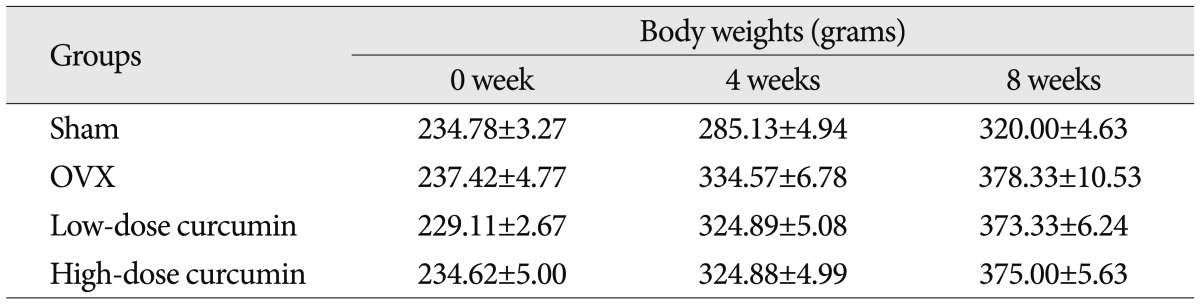
Body weights were measured once a week throughout the 8-week experiment period. Table 1 shows mean body weights of the 40 rats euthanized 8 weeks after OVX or sham operations. At the start of the experiment, body weights were similar in all groups. However, at 4 weeks after surgery, the mean body weight in the untreated OVX group and treated groups (low-dose and high-dose curcumin group) was significantly greater than in the sham group, and this significant difference was maintained throughout the experimental period (p<0.05 at 4 and 8 weeks). The mean body weight in the untreated OVX group and treated groups (low-dose and hig-dose curcumin group) were similar throughout the experiment period.
Serum estrogen (estradiol, E2) levels were expressed as percentage of the serum levels of the sham groups (Fig. 1). As expected, serum estrogen levels in the untreated OVX group were significantly decreased after ovariectomy (p<0.05 at 4 and 8 weeks). Serum estrogen levels in low-dose curcumin treated group were slightly decreased compared than that of the sham group, but, which were not statistically significant (p>0.05). Also, serum estrogen levels in the high-dose curcumin treated group were slightly higher compared to that of the sham group, which were not statistically significant (p>0.05).
Fig. 2A, B show the serum levels of the bone formation biochemical markers, osteocalcin and ALP, and Fig. 2C shows the serum levels of CTX-1, which is a sensitive marker of bone resorption. Results have been expressed as percentage of the serum levels of the biochemical markers of the sham group.
As expected, in the untreated OVX group, serum levels of osteocalcin, ALP, and CTX-1 were significantly increased compared than that of the sham group.
At 4 and 8 weeks after surgery, the concentrations of the bone formation markers (osteocalcin and ALP) in the curcumin administered groups were significantly lower than in the untreated OVX group. Compared with the low-dose curcumin group, the high-dose curcumin group had lower osteocalcin and ALP levels at 8 weeks, statistically significant differences.
As shown in the graph of the concentrations of CTX-1 (Fig. 2C), the low-dose curcumin group had similar serum levels of CTX-1 compared than that of the sham group. In contrast, the high-dose curcumin group had significantly lower CTX-1 concentration at 4 and 8 weeks compared with the sham group as well as with the low-dose curcumin group.
As shown in the representative micro-CT images of the 4th lumbar spine (Fig. 3), the untreated OVX rats had fewer trabecular bone structures than that of the sham controls at 8 weeks after ovariectomy. The high-dose curcumin group had more trabecular bone structures compared with the low-dose curcumin group as well as the untreated OVX group.
Fig. 4 shows the changes of bone histomorphometric parameters of the 4th lumbar vertebra 8 weeks after ovariectomy. All values represent 100% minus the percentage of the value of the sham group/the value of the experimental groups.
In the analyses of micro-CT scans of 4th lumbar vertebrae, the curcumin treated groups (low-dose and high-dose curcumin groups) showed a sustained increase in spine BMD and CrBMD compared with the untreated OVX group. The high-dose curcumin treated group had a significant increase in BMD (p=0.028) and CrBMD (p=0.036) compared with the low-dose curcumin treated group.
Considering BV/TV, the curcumin treated groups (low-dose and high-dose curcumin groups) showed a significant increase compared with the untreated OVX group (p=0.035 and p=0.001). The high-dose curcumin group showed a sustained increase compared with the low-dose curcumin treated group, however, which was not statistically significant (p=0.077).
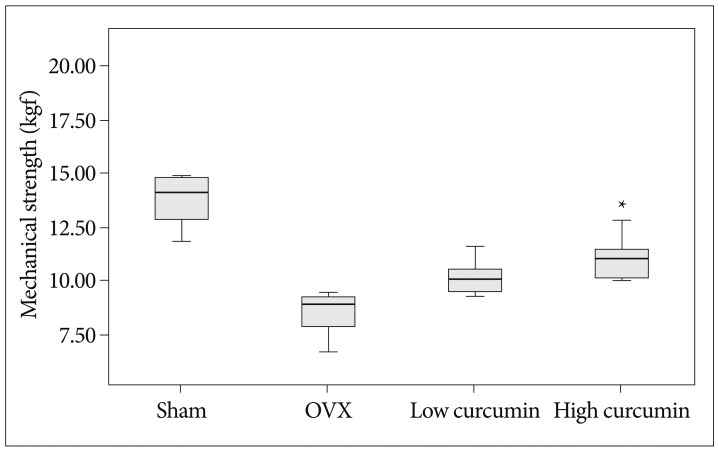
Although the three point bending test showed that the 4th lumbar vertebrae of the low-dose and high-dose curcumin treated groups had a greater maximal load value compared to the untreated OVX group, the only high-dose curcumin treated group differed significantly from the untreated OVX group (p=0.015) (Fig. 5).
Although bisphosphonates, potent antiresorptive agents, have been the most popular first-line drugs for the treatment of osteoporosis for some time, many side effects have become evident in recent years4,8,18,21). Some reports suggested a link between BPs use and the development of atypical insufficiency fractures or osteonecrosis of the jaw14,15). These are thought to be due to long term oversuppression of bone turnover leading to impaired bone remodeling, accumulation of microdamage in bone and increased skeletal fragility. Therefore, there is increasing interest in the discovery of natural substances that could favorably affect the skeletal system, which could be used in place of pharmacological treatment for osteoporosis.
Curcumin is a nonsteroidal, naturally occurring compound in the rhizomes of the popular Indian spice turmeric plant (Curcumin longa L.), which is commonly used as a dietary pigment. In addition to a variety of pharmacologic effects, including anti-inflammatory, anti-infectious and antioxidant activities, which are traditionally known5,13,19), recent studies investigated the protective effects of curcumin on the regulation of bone remodeling. It is well known that curcumin has action similar to bisphosphonate, the inhibition of osteoclastogenesis4-7,9,23). Ozaki et al.10) showed that curcumin is a potent stimulator of osteoclast apoptosis and also an inhibitor of bone resorption caused by rabbit osteoclast. It has also been shown in murine cells that curcumin inhibits osteoclastogenesis induced by receptor activation of NF-kB ligand2).
In the study by Folwarczna et al.5), 10 mg/kg curcumin was administered po daily for 4 weeks to normal and bilaterally ovariectomized rats. In their study, although curcumin slightly improved some bone histomorphometric parameters impaired by estrogen deficiency, the effects on the skeletal system were ambiguous. They concluded that curcumin at a dietary achievable dose may not be useful for the prevention or treatment of osteoporosis.
Curcumin has been consumed as a dietary spice at doses of up to 100 mg/day19), i.e., approximately 1.5 mg/kg/day, assuming that human body mass is 65-70 kg. According to Phase I clinical trials, humans can tolerate curcumin even at a dose of 8 g/day2).
Because it is known that the oral bioavailability of curcumin in rats is about 1% and the half-life of curcumin is rather short22), we hypothesized that a relatively high concentration of curcumin would have a protective effect of bone remodeling. Thus in the present study, ovariectomized rats were treated either with low-dose (10 mg/kg) or high-dose (50 mg/kg) curcumin. We then compared the therapeutic effects on bone remodeling with different dose of curcumin treatment using bone turnover markers, histomorphometric parameters and bone strength.
French et al.6) conducted a well-designed experiment about the bone sparing effect of curcumin or bisphosphonate (etidronate) in the ovariectomized rat. They used three different doses of curcumin; 1.5 mg/kg, 3 mg/kg, 15 mg/kg, which are a relatively low concentration of curcumin compared with the doses of our study. In their study, there was no difference in lumbar spine BMD at two months after ovariectomy. At four months post-ovariectomy, all three curcumin groups demonstrated a sustained increase in spine BMD over ovariectomized animals, which was not statistically significant. There was a 50% increase in mechanical strength for all groups of animals that received curcumin. We think that this lack of a significant increase in spine BMD in the curcumin group in the study by French et al.6) may be due to administration of a dose of curcumin that was too low to produce a significant increase in spine BMD.
In our study using a relatively high dose of curcumin, the curcumin treated groups (low-dose and high-dose curcumin groups) showed a sustained increase in spine BMD and CrBMD compared with the untreated OVX group. In the comparison between the different doses of curcumin, the high-dose curcumin treated group had a significant increase in BMD and CrBMD compared with the low-dose curcumin treated group. Considering mechanical strength, the low-dose and high-dose curcumin treated groups had a greater maximal load value compared to the untreated OVX group, but only high-dose curcumin treated group had a significant difference from the untreated OVX group (p=0.015). Thus our study supported the results of Folwarczna et al.5). We strongly agree with their opinion, that curcumin at a dietary achievable dose may not be useful for the prevention or treatment of osteoporosis. We think high-dose curcumin treatment could lead to more advantages in the bone sparing effect.
As mentioned above, curcumin has an action similar to bisphosphonate, the inhibition of bone resorption by osteoclast. Biochemical markers of bone turnover have been widely used as measures of the status of bone remodeling. The extent of bone resorption could be checked by CTX-1 level (a sensitive marker of bone resorption). French et al.6) noted that the curcumin treated group showed CTX concentrations very similar to those of ovariectomized animals given etidronate. In our previous study about a synergistic bone sparing effect of curcumin (50 mg/kg, daily) and alendronate in ovariectomized rats, we achieved results comparable to French et al.6) CTX-1 concentration in curcumin administered group was similar to those of the alendronate administered group. In addition, the combination therapy (50 mg/kg curcumin and alendronate) group had lower CTX-1 concentrations, which were statistically significant to the curcumin only and the alendronate only group3).
In the present study, the high-dose curcumin group had significantly lower CTX-1 concentration compared with the sham group and the low-dose curcumin group. This is further evidence of our hypothesis that high-dose curcumin treatment could lead to better bone sparing effect.
References
1. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007; 22:465–475. PMID: 17144789.

2. Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001; 21:2895–2900. PMID: 11712783.
3. Cho DC, Kim KT, Jeon Y, Sung JK. A synergistic bone sparing effect of curcumin and alendronate in ovariectomized rat. Acta Neurochir (Wien). 2012; 154:2215–2223. PMID: 23053289.

4. Fleisch H. Bisphosphonates : mechanisms of action. Endocr Rev. 1998; 19:80–100. PMID: 9494781.
5. Folwarczna J, Zych M, Trzeciak HI. Effects of curcumin on the skeletal system in rats. Pharmacol Rep. 2010; 62:900–909. PMID: 21098873.

6. French DL, Muir JM, Webber CE. The ovariectomized, mature rat model of postmenopausal osteoporosis : an assessment of the bone sparing effects of curcumin. Phytomedicine. 2008; 15:1069–1078. PMID: 18693096.

7. Kashii M, Hashimoto J, Nakano T, Umakoshi Y, Yoshikawa H. Alendronate treatment promotes bone formation with a less anisotropic microstructure during intramembranous ossification in rats. J Bone Miner Metab. 2008; 26:24–33. PMID: 18095060.

8. Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA, Pak CY. Severely suppressed bone turnover : a potential complication of alendronate therapy. J Clin Endocrinol Metab. 2005; 90:1294–1301. PMID: 15598694.

9. Oh S, Kyung TW, Choi HS. Curcumin inhibits osteoclastogenesis by decreasing receptor activator of nuclear factor-kappaB ligand (RANKL) in bone marrow stromal cells. Mol Cells. 2008; 26:486–489. PMID: 18719352.
10. Ozaki K, Kawata Y, Amano S, Hanazawa S. Stimulatory effect of curcumin on osteoclast apoptosis. Biochem Pharmacol. 2000; 59:1577–1581. PMID: 10799655.

11. Palumbo C, Ferretti M, Bertoni L, Cavani F, Resca E, Casolari B, et al. Influence of ferutinin on bone metabolism in ovariectomized rats. I: role in preventing osteoporosis. J Bone Miner Metab. 2009; 27:538–545. PMID: 19333679.

12. Park SB, Lee YJ, Chung CK. Bone mineral density changes after ovariectomy in rats as an osteopenic model : stepwise description of double dorso-lateral approach. J Korean Neurosurg Soc. 2010; 48:309–312. PMID: 21113356.

13. Park SK, Oh S, Shin HK, Kim SH, Ham J, Song JS, et al. Synthesis of substituted triazolyl curcumin mimics that inhibit RANKL-induced osteoclastogenesis. Bioorg Med Chem Lett. 2011; 21:3573–3577. PMID: 21570847.

14. Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL. Osteonecrosis of the jaws associated with the use of bisphosphonates : a review of 63 cases. J Oral Maxillofac Surg. 2004; 62:527–534. PMID: 15122554.

15. Schneider JP. Should bisphosphonates be continued indefinitely? An unusual fracture in a healthy woman on long-term alendronate. Geriatrics. 2006; 61:31–33. PMID: 16405362.
16. Sheng ZF, Dai RC, Wang P, Yao XF, Feng XQ, Fang LN, et al. [Nanomechanical properties of vertebral trabeculae in ovariectomized rats]. Zhonghua Yi Xue Za Zhi. 2006; 86:515–519. PMID: 16681878.
17. Shin YH, Cho DC, Yu SH, Kim KT, Cho HJ, Sung JK. Histomorphometric analysis of the spine and femur in ovariectomized rats using micro-computed tomographic scan. J Korean Neurosurg Soc. 2012; 52:1–6. PMID: 22993670.

18. Shiraishi A, Miyabe S, Nakano T, Umakoshi Y, Ito M, Mihara M. The combination therapy with alfacalcidol and risedronate improves the mechanical property in lumbar spine by affecting the material properties in an ovariectomized rat model of osteoporosis. BMC Musculoskelet Disord. 2009; 10:66. PMID: 19527501.

19. Shishodia S, Sethi G, Aggarwal BB. Curcumin : getting back to the roots. Ann N Y Acad Sci. 2005; 1056:206–217. PMID: 16387689.
20. Szulc P, Delmas PD. Biochemical markers of bone turnover : potential use in the investigation and management of postmenopausal osteoporosis. Osteoporos Int. 2008; 19:1683–1704. PMID: 18629570.

21. Watts NB, Diab DL. Long-term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab. 2010; 95:1555–1565. PMID: 20173017.

22. Yang KY, Lin LC, Tseng TY, Wang SC, Tsai TH. Oral bioavailability of curcumin in rat and the herbal analysis from Curcuma longa by LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2007; 853:183–189.

23. Yang MW, Wang TH, Yan PP, Chu LW, Yu J, Gao ZD, et al. Curcumin improves bone microarchitecture and enhances mineral density in APP/PS1 transgenic mice. Phytomedicine. 2011; 18:205–213. PMID: 20637579.

Fig. 1
Graph showing the serum levels of estrogen (estradiol, E2). Values are expressed as percentage of the serum levels of the sham group. *p<0.05 for the values of the untreated ovariectomy group compared with that of the sham group.
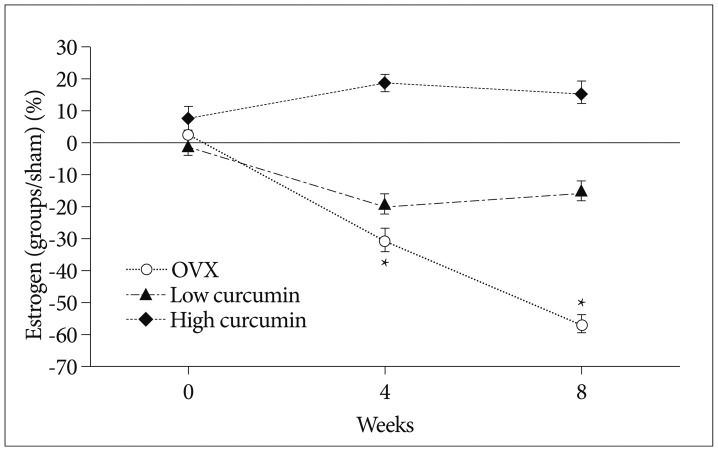
Fig. 2
Graphs showing the serum levels of osteocalcin (A), alkaline phosphatase (ALP) (B), and type I collagen C-telopeptide (CTX-1) (C). Values are expressed as percentage of the serum levels of the sham group. *p<0.05 for the values of the low-dose or high-dose curcumin group compared with that of untreated ovariectomy (OVX) group, †p<0.05 for the values of the high-dose curcumin group compared with that of low-dose curcumin group.
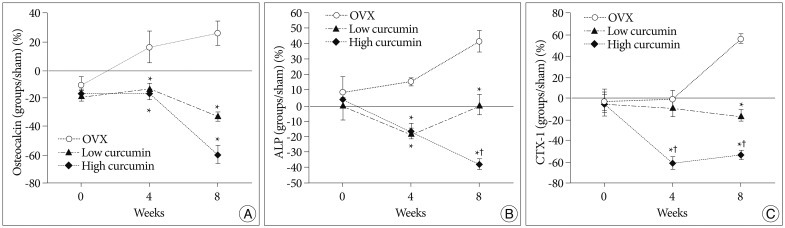
Fig. 3
Representative micro-CT images of the 4th lumbar spine in the sham and experimental groups. The high-dose curcumin group had more trabecular bone structures compared with the low-dose curcumin group as well as the untreated ovariectomy (OVX) group.
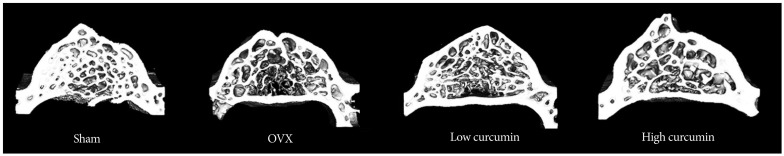
Fig. 4
Bar graph showing the result of histomorphometric analyses of 4th lumbar vertebrae in the sham and experimental groups at 8 weeks post-operation : bone mineral density (BMD) (A), cortical bone mineral density (CrBMD) (B), trabecular bone volume fraction (BV/TV) (C). All values represent 100% minus the percentage of the value of the sham group/the value of the experimental groups. *p<0.05 for the values of the low-dose or high-dose curcumin group compared with that of untreated ovariectomy (OVX) group, and †p<0.05 for the values of the high-dose curcumin group compared with that of low-dose curcumin group.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download