Abstract
Objective
Use of quantitative computed tomography (CT) to evaluate bone mineral density was suggested in the 1970s. Despite its reliability and accuracy, technical shortcomings restricted its usage, and dual-energy X-ray absorptiometry (DXA) became the gold standard evaluation method. Advances in CT technology have reduced its previous limitations, and CT evaluation of bone quality may now be applicable in clinical practice. The aim of this study was to determine if the Hounsfield unit (HU) values obtained from CT correlate with patient age and bone mineral density.
Methods
A total of 128 female patients who underwent lumbar CT for back pain were enrolled in the study. Their mean age was 66.4 years. Among them, 70 patients also underwent DXA. The patients were stratified by decade of life, forming five age groups. Lumbar vertebrae L1-4 were analyzed. The HU value of each vertebra was determined by averaging three measurements of the vertebra's trabecular portion, as shown in consecutive axial CT images. The HU values were compared between age groups, and correlations of HU value with bone mineral density and T-scores were determined.
Osteoporosis is a systemic skeletal disease characterized by low bone density and microarchitectural bone tissue deterioration with a consequent increase in bone fragility12). Approximately 100 million people are affected by osteoporosis worldwide, mostly women in menopause6). In 2008, about 40% of women in Korea were reported to have osteoporosis7). As the older age groups in population increase due to an increase of life expectancy, the socioeconomic burden of this disease increases, particularly as bone fracture risk increases four- to six-fold among those with osteoporosis13). Dual-energy X-ray absorptiometry (DXA), the gold standard for bone mineral density quantification, has become a routine screening in modern medical practice.
Measuring bone mineral density by using quantitative computed tomography (CT) was suggested in the 1970s1). However, shortcomings such as the requirements for a high dose of ionizing radiation and a relatively long scanning time and introduction of DXA, resulted in quantitative CT usage being confined to musculoskeletal research fields despite its early introduction and accuracy. Recently, diagnostic imaging technology has been advancing rapidly, and the use of CT has been remarkably increasing in clinical fields, both in extent and numbers4). With intraobtechnical developments in CT systems, the advantages of CT over DXA have drawn attention for the second time.
Recent studies have raised the possibilities to estimate bone mineral density using diagnostic CT images8-11,15). It is very early to say but Hounsfield unit (HU) values might be a surrogate marker for bone mineral density. In this study, we investigated whether there was a correlation between the bone mineral density and T-scores measured by DXA and the HU values obtained from diagnostic CT scanners equipped with automatic exposure control technology. Based on this work, we might find a way to use diagnostic CT images to screen the patients with bone mineral disease and to evaluate their risks of fractures.
After obtaining approval from our institutional review board, we retrospectively reviewed female patients with low back pain who had undergone lumbar CT with thin slice sections at our institution between 2010 and 2011. All reviewed patients were over 40 years old. DXA scan within twelve months from CT scan were used for correlation analysis. Patients who underwent previous spine surgeries with spinal instrument implantation or vertebroplasty, or invalid DXA results due to spinal degeneration, fracture, or deformity (as evaluated by authors) were excluded from the study. Of the approximately 2000 subjects reviewed, 128 patients were enrolled in this study.
All DXA scans were performed with use of a Lunar Prodigy Advance densitometer (General Electric, Milwaukee, WI, USA). Information from DXA scans, including T-scores and bone mineral density (measured in g/cm2), were obtained for the first through fourth lumbar vertebrae.
For CT scans, a helical 128-channel CT scanner (Ingenuity; Phillips Healthcare, Best, the Netherlands) was utilized for all patients. The CT parameters included a slice thickness of 1.0 mm, a tube voltage of 120 kVp, a tube current of 330 mA (DoseRight automated exposure control system; Phillips), and a bone reconstruction algorithm (window width/window level, 2050/250). Two-dimensional reconstructions were obtained in the coronal and sagittal planes. Phantoms were not used during CT scan.
A picture archiving and communication system (PACS; Maroview, Infinitt Healthcare), which operated in a Microsoft Windows environment, was used to calculate an average HU value for an elliptical region of interest that was confined to the trabecular area of the vertebral body. The HU measurement for each vertebra was obtained by using a protocol described by Schreiber et al.14). Regions of interest were measured on the axial images at L1 through L4 at three separate locations : immediately inferior to the superior end plate, in the middle of the vertebral body, and superior to the inferior end plate (Fig. 1). For each measurement, the largest possible elliptical region of interest was drawn, excluding the cortical margins to prevent volume averaging. The HU values from the three axial slices were averaged to give a mean HU value for each lumbar vertebra. Measurements were obtained twice by one observer independently and with one week separating observations. During the measures, the observer was blinded to the patients' DXA scores. The average of the two independent measures was used during analysis.
An intraobserver reliability calculation was performed with the use of the interclass correlation coefficient and was reported as a score between 0 and 1 (with 0 representing no agreement and 1 representing perfect agreement). The difference in HU values between age groups was evaluated with the use of one-way analysis of variance (ANOVA). Fisher's least significant difference multiple t-tests were used for post hoc analysis. The correlations of HU value with age group and with DXA bone mineral density and T-score were determined with the use of the Pearson correlation coefficient.
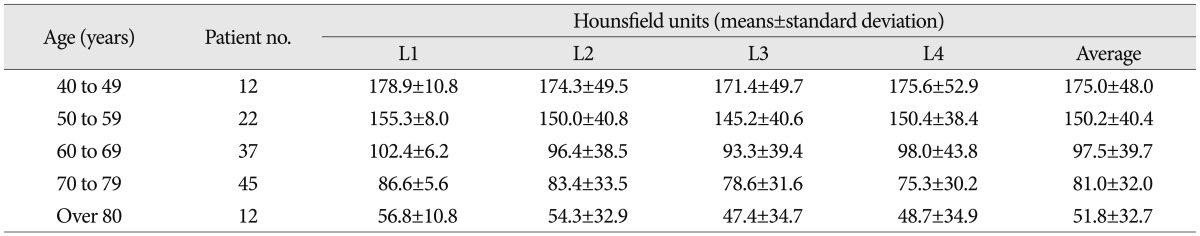
The HU values obtained from CT for the 128 patients included in this study were stratified by decade of life, forming five age groups. The HU values for each vertebral level were compared between the groups. Mean HU values decreased consistently by decade at all compared vertebral levels, ranging from a mean of 175.0 HU in the 5th decade of life to 51.8 HU in the 9th decade of life (Table 1). The differences were significant (one-way ANOVA, p<0.05). Subgroup analysis was also performed. In the L1-3 vertebrae, there were no significant differences between the 40s and 50s or 60s and 70s age groups; however, there were significant differences between the 50s and 60s groups and the 70s and 80s groups (p<0.05) (Fig. 2). Moreover, there was a significant HU decrease between the 60s and 70s age groups in the L4 vertebra (p<0.05).
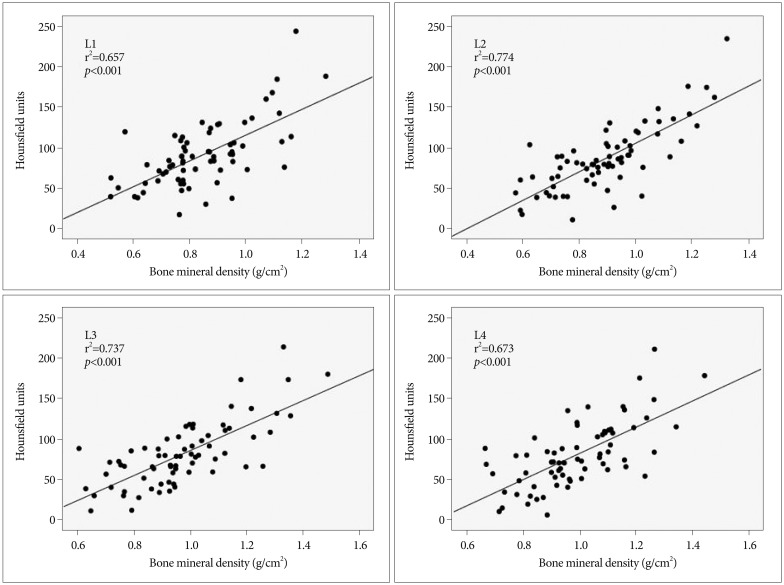
Among the enrolled patients, 70 patients underwent DXA within one year of their CT scan. Mean age of those 70 patients was 71.2 years (range, 53-87 years). The average L1-4 HU values for the 70 patients ranged from 11.4 HU to 226.2 HU (mean with standard deviation, 84.7±40.1) while their T-scores ranged from -4.4 to +1.6 (mean, -1.7±1.49), and their bone mineral density ranged from 0.616 to 1.33 g/cm2 (mean, 0.925±0.183 g/cm2). The correlations of HU value with bone mineral density and T-score were evaluated for each lumbar vertebra separately. The correlation coefficients (r2) between the HU value and T-score were for the L1-4 vertebrae were 0.673, 0.794, 0.766, and 0.713, respectively (Fig. 3), while the r2 between the HU value and bone mineral density for the L1-4 vertebrae were 0.657, 0.774, 0.737, and 0.673, respectively (Fig. 4). All obtained correlations were significant (p<0.001).
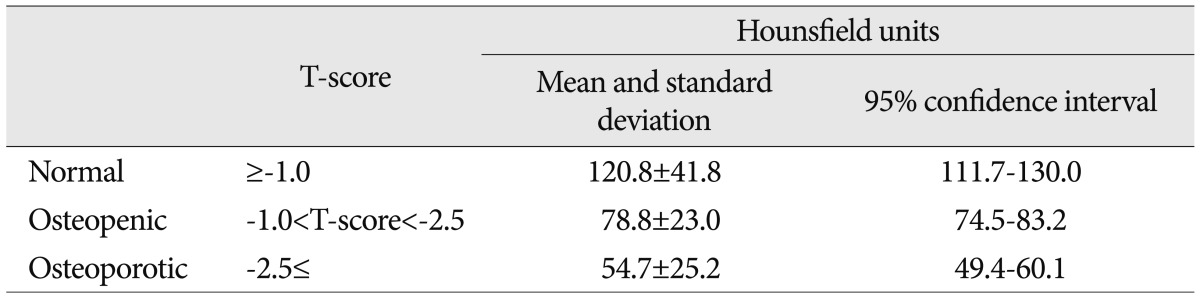
Based on the World Health Organization's guideline12), the 70 patients' lumbar vertebrae T-scores were stratified into three groups : normal (-1.0 or greater), osteopenic (less than -1.0 and greater than -2.5), and osteoporotic (-2.5 or less). The mean HU values for the subjects in the normal, osteopenic, and osteoporotic groups were 120.8 HU (95% confidence interval, 111.7-130.0), 78.8 HU (95% confidence interval, 74.5-83.2), and 54.7 HU (95% confidence interval, 49.4-60.1), respectively (Table 2). The differences in mean HU values between groups were all significant (p<0.001).
Through this study, we have determined that the bone mineral density measurement provided by DXA has a strong correlation with the HU value obtained by using CT with automatic exposure control. The HU scale is a linear transformation of the original linear attenuation coefficient measurement into one in which the radiodensity of distilled water at a standard pressure and temperature is defined as zero HU. With µwater and µx representing the total linear X-ray attenuation coefficient of distilled water and a selected voxel, respectively, the corresponding standardized HU value of the voxel is generated by : CT value (HU) =1000×[(µx-µwater)/µwater]. On that basis, and in all appropriately calibrated CT scanners, particular portion of a CT image can be assigned a specific HU value with validity and reproducibility3). Previous biomechanical study has shown that an increase in HU value is correlated linearly with an increase in material density14). In our study, HU values followed a similar trend. In addition, HU values showed a decreasing trend with increasing age and with a decrease in bone mineral density.
Quantitative CT requires the use of a calibration phantom for which the density is known. The phantom scanned with the patient in order to convert HU values into bone mineral units and to permit calibration of other factors that may result errors1). Recent progress in CT technology has developed an auto-modulation technique, also known as automatic exposure control, which limits the amount of radiation to that required for image acquisition. The CT scanner utilizes data from the scout view as well as real-time feedback from the detectors to determine the necessary exposure time and tube current. Based on those data, a specific amount of X-rays will accumulate to a pre-set threshold, after which X-ray exposure terminates. The amount of radiation exposure decreases for less dense regions of the body, and increases for denser portions of the body. This results in a more homogenous X-ray beam energy spectrum encountered by the spine, thus making the obtained HU values primarily dependent on the composition of the targeted tissue. As a patient's body-mass index accounts for a large portion of the attenuation differences, the use of an automatic exposure control technique theoretically could eliminate the use of phantoms during CT calibration14).
To establish a diagnosis of osteoporosis, DXA-based T-scores, defined by the number of standard deviations below the mean peak bone mass (average mass of young, healthy adults), are used by physicians. In present study, HU values were found to be significantly correlated to T-scores. Thus, a patient's HU values may provide a physician with an indication of osteoporosis presence, and may encourage the physician to undertake further studies including DXA and proper patient management. In this type of CT-based screening, it would be helpful to know the range of HU values that match with the presence of osteoporosis. In the present study, subjects with a normal bone density had a mean lumbar HU value of 120.8, those with osteopenia had a mean lumbar HU value of 78.8, and those with osteoporosis had a mean lumbar HU value of 54.7. Such values could be used as reference points about which suitable ranges could be established.
CT scans are one of the most popular diagnostic tools in modern medical practice. A fairly large number of spine CT scans is being performed at many institutes. In addition to the imaging protocol of the spine itself, there are a number of other protocols that cover the spine, such as chest, abdomen, pelvis and genitourinary and angiography covering the thoracoabdominal area, etc. Thus, compared to DXA, which is performed only on high risk patients such as post-menopausal women, CT provides much more data that can be used in the evaluation of the bone mineral density of the spine. Patients in various age groups regardless of sex can be evaluated without any additive cost. According to the data provided in this study, a patient with an average HU value below 60 has a high probability of osteoporosis. A confirmatory diagnosis is not yet possible due to the lack of data that correlates fracture risks and HU values in the general population. HU values could be very practical and useful for screening purposes.
However, the reference data of our study came from one CT scanner with one automated exposure control technique and one DXA, there may be a question about the reproducibility of HU values obtained from different CT and DXA systems. Unlike their use in quantitative CT for bone mineral density measurement, phantoms were not used in this study. In general, the zero HU value was set to the radiodensity of distilled water at standard pressure and temperature and the CT system's automated exposure control delivered the same amount of radiation dose to the lumbar spine regions of interest. Theoretically, these settings allow us to acquire a constant HU value for a certain vertebra regardless of the CT device used or the patient's body mass14). But still, this study has certain limitation since it has no specific data from other CT and DXA system to prove our premise.
Moreover, our study has several limitations. First, the data obtained for correlations between the HU value and the age groups were only from female patients aged over 40 years. This may bias the results so they may not accurately extrapolate to other population groups. Second, the maximum one year interval between CT and DXA may have influenced the results. However, we suggest this is unlikely unless the patients are under medical treatment for osteoporosis or suffering from an endocrine disorder that affects bone mineral density. Third, cancellous bone is heterogeneous; therefore three axial sections may not accurately summarize bone quality5). Fourth, only trabeculated bone density was included in the HU values obtained from CT while DXA assesses both cancellous and cortical bone. Although cancellous bone has been shown to be more important than cortical bone for vertebral load sharing and fracture risk2), this difference in bone types may have resulted in errors in the correlation between DXA the bone mineral density and CT HU value.
The diagnostic CT-based HU value and DXA-based bone mineral density showed strong positive correlation. By measuring HU value from CT scanners with automated exposure control, we may be able to estimate bone mineral density. Considering the abundance of data, and the simplicity, reliability and reproducibility of the measurement, the diagnostic CT-based HU value could be helpful in the screening of bone metabolic diseases.
References
2. Eswaran SK, Gupta A, Adams MF, Keaveny TM. Cortical and trabecular load sharing in the human vertebral body. J Bone Miner Res. 2006; 21:307–314. PMID: 16418787.

3. Goldman LW. Principles of CT and CT technology. J Nucl Med Technol. 2007; 35:115–128. quiz 129-130. PMID: 17823453.

4. Goo HW. CT radiation dose optimization and estimation : an update for radiologists. Korean J Radiol. 2012; 13:1–11. PMID: 22247630.

5. Keaveny TM, Hayes WC. A 20-year perspective on the mechanical properties of trabecular bone. J Biomech Eng. 1993; 115:534–542. PMID: 8302037.

6. Kim DH, Vaccaro AR. Osteoporotic compression fractures of the spine; current options and considerations for treatment. Spine J. 2006; 6:479–487. PMID: 16934715.

7. Kim KH, Lee K, Ko YJ, Kim SJ, Oh SI, Durrance DY, et al. Prevalence, awareness, and treatment of osteoporosis among Korean women : The Fourth Korea National Health and Nutrition Examination Survey. Bone. 2012; 50:1039–1047. PMID: 22366398.

8. Link TM, Koppers BB, Licht T, Bauer J, Lu Y, Rummeny EJ. In vitro and in vivo spiral CT to determine bone mineral density : initial experience in patients at risk for osteoporosis. Radiology. 2004; 231:805–811. PMID: 15105454.

9. Miyabara Y, Holmes D 3rd, Camp J, Miller VM, Kearns AE. Comparison of calibrated and uncalibrated bone mineral density by CT to DEXA in menopausal women. Climacteric. 2012; 15:374–381. PMID: 22175297.

10. Papadakis AE, Karantanas AH, Papadokostakis G, Damilakis J. Assessment of the morpho-densitometric parameters of the lumbar pedicles in osteoporotic and control women undergoing routine abdominal MDCT examinations. J Bone Miner Metab. 2011; 29:352–358. PMID: 20976512.

11. Papadakis AE, Karantanas AH, Papadokostakis G, Petinellis E, Damilakis J. Can abdominal multi-detector CT diagnose spinal osteoporosis? Eur Radiol. 2009; 19:172–176. PMID: 18641992.

12. Prevention and management of osteoporosis. World Health Organ Tech Rep Ser. 2003; 921:1–164. PMID: 15293701.
13. Ross PD, Davis JW, Epstein RS, Wasnich RD. Pre-existing fractures and bone mass predict vertebral fracture incidence in women. Ann Intern Med. 1991; 114:919–923. PMID: 2024857.

14. Schreiber JJ, Anderson PA, Rosas HG, Buchholz AL, Au AG. Hounsfield units for assessing bone mineral density and strength : a tool for osteoporosis management. J Bone Joint Surg Am. 2011; 93:1057–1063. PMID: 21655899.

15. Tay WL, Chui CK, Ong SH, Ng AC. Osteoporosis screening using areal bone mineral density estimation from diagnostic CT images. Acad Radiol. 2012; 19:1273–1282. PMID: 22958722.

Fig. 1
Computed tomography (CT) scans of lumbar vertebra L3 illustrating the method of determining Hounsfield unit (HU) values by using a picture archiving and communication system (PACS). From a reconstructed sagittal image, we select three axial planes of interest : slice (A) is taken just inferior to the superior endplate, slice (B) is from the middle of the vertebral body, and slice (C) is taken just superior to the inferior endplate. The PACS program automatically calculates the mean HU value of the regions of interest which are marked with ellipses in the figure. The average of HU values from three axial cuts, which is 89 HU in this case, was used for the analysis.
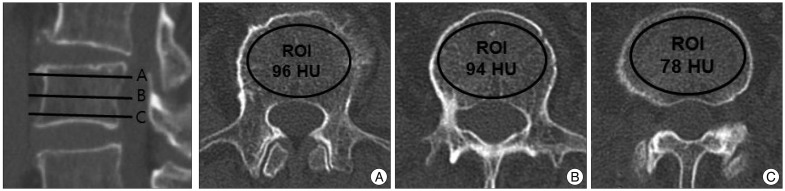
Fig. 2
Mean Hounsfield unit values among five decadal age groups show consistent decreases as age increases. *Groups which showed significant difference (p<0.05).
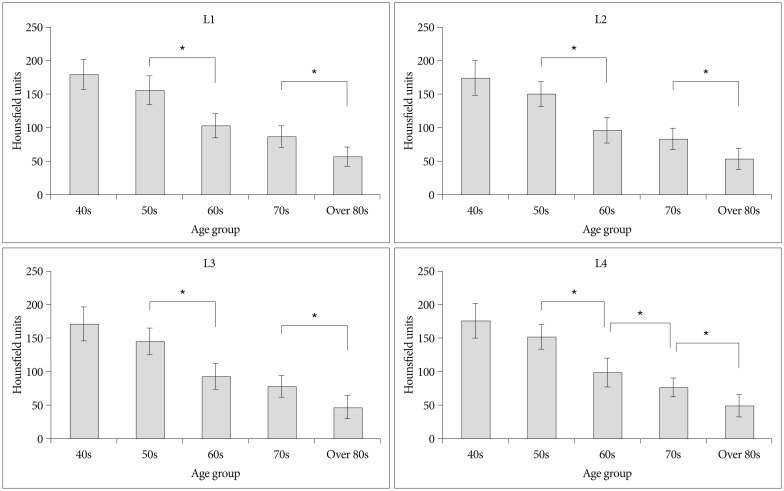
Fig. 3
Scatter plots showing correlations between Hounsfield unit values obtained from CT and T-scores obtained from dual-energy X-ray absorptiometry for lumbar vertebrae L1-L4. All had showed significant correlation coefficients (p<0.001).

Fig. 4
Scatter plots showing correlations between Hounsfield unit values obtained from CT scans and bone marrow density obtained from dual-energy X-ray absorptiometry for lumbar vertebrae L1-4. All had significant correlation coefficients (p<0.001).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download