Abstract
To present a rare case of a cystic giant schwannoma of the sacrum mimicking aneurysmal bone cyst (ABC). A 54-year-old man visited our institute complaining left leg weakness and sensory change for several years. Magnetic resonance imaging revealed a large multilocular cystic mass with canal invasion and bone erosion confined to left S1 body. The lesion showed multiple septal enhancement without definite solid component. Initially the tumor was considered as ABC. The patient underwent grossly-total tumor resection with lumbosacral reconstruction via posterior approach. The tumor was proved to be a cystic schwannoma. The postoperative course was uneventful and the patient was relieved from preoperative symptoms. We present a rare case of pure cystic giant schwannoma confined to sacrum mimicking ABC. The surgical treatment is challenging due to the complex anatomy of the sacrum. Schwannoma should be considered in the differential diagnosis of osteolytic sacral cysts.
Spinal schwannoma are relatively common, accounting for 25% of all primary spinal tumors3,6). Most of spinal shwannomas are small, well-circumscribed, benign tumors.
The incidence of sacral schwannoma ranges from 1% to 5% of all spinal schwannomas and only about 50 cases are reported in the literature4). These sacral tumors tend to reach a considerable size before symptomatic presentation owing to their insidious and slow growing characteristics, resulting extensive bone erosion1,19,22). The mobility of nerve roots and wide capacity of sacral canal also aid in extensive tumor growth.
Most schwannomas are solid or heterogenous-solid tumors and degenerative changes into hemorrhage, calcification, and fibrosis are rather common. However, cystic changes are very rare23).
A 54-year-old man visited the outpatient clinic complaining tingling sensation and paresthesia of left lower limb for several years. His symptoms have been aggravated during the last several months and there has been no improvement after conservative cares. He had no history of previous trauma or constitutional symptoms. Neurologic examination revealed weakness of dorsiflexion and plantaflexion on the left foot with mild hypesthesia at the left S1 dermatome. Perineal and perianal sensations were also mildly decreased.
Plain radiograph showed an osteolytic lesion limited to left sacral ala sparing lumbar spine (Fig. 1). Magnetic resonance (MR) imaging of lumbar spine revealed about 5.1×3.4×3.6 cm sized multilocular cystic mass with canal invasion and bone erosion of the left S1 body. The mass showed homogenous hypointense signal on T1-weighted image and hyperintense signal on T2-weighted image. The lesion showed multi-septated cystic rim enhancement after administration of gadolinium contrast, but no definite solid portion was found. The left S1 root was also obliterated, which seemed to be main cause of leg symptoms. No extraspinal component was identified (Fig. 2). Computer tomography (CT) myelography was performed and intraosseous expensile mass involving left sacrum was presented along with left S1 root compression and displacement of the left sacral nerves below. A subtle pathologic fracture involving upper endplate of S1 was also noted (Fig. 3).
The initial preoperative diagnosis was ABC, with several radiologic clues such as the location of mass, which was presenting in sacrum and compromising the neural arch, near totally cystic nature and balloon-like expanslile remodeling of bone. Even fluid-fluid level was not revealed in the imaging studies, ABC was strongly suspected.
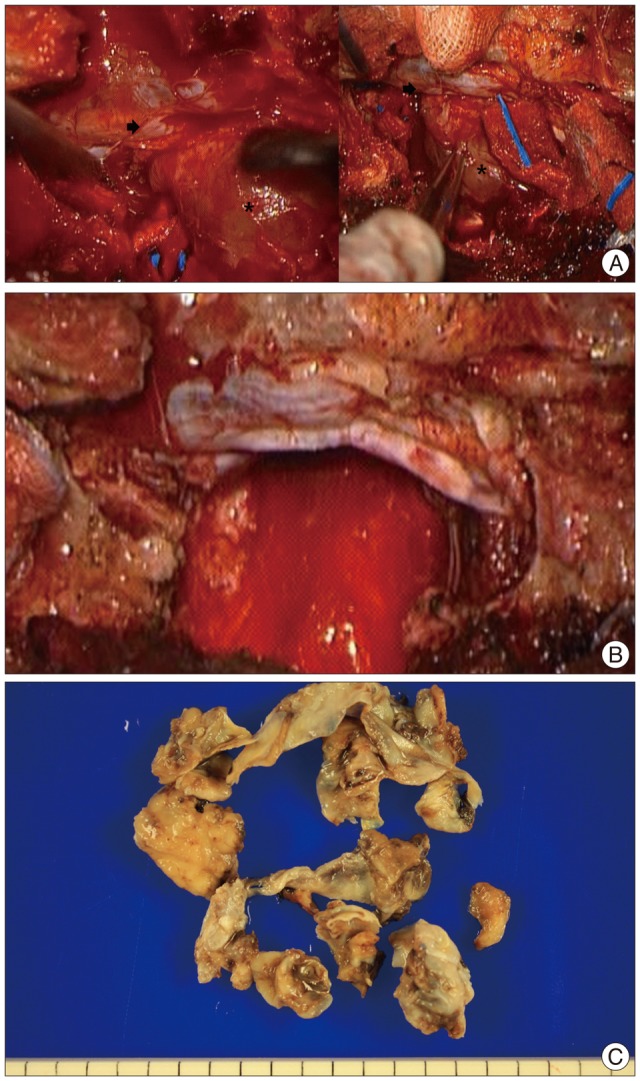
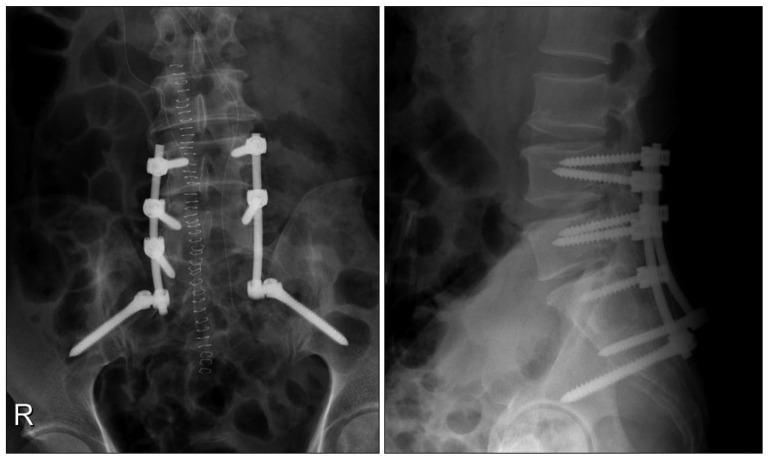
We underwent a grossly-total tumor resection with lumbosacral reconstruction via posterior approach. A well-defined, encapsulated, cystic mass was found in epidural space adherent to nerve sheath displacing sacral nerve roots peripherally. The borders of tumor was carefully identified and dissected to preserve capsule of the cystic component under operating microscope. Once pericapsular dissection was done, en-bloc tumor resection was followed and sacral nerve roots were successfully preserved under spontaneous and evoked electromyography monitoring (Fig. 4). Since more than half of S1 body had been eroded by the tumor, an instrumented stabilization with fixation from L4 to the ilium (iliac screws) was performed (Fig. 5).
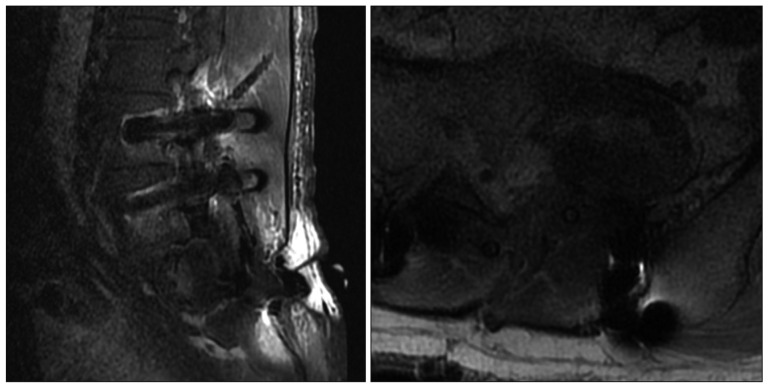
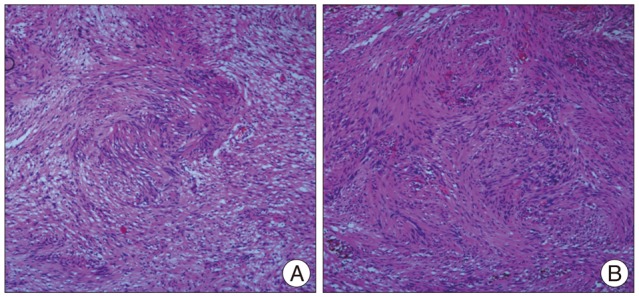
After the operation, the patient was completely recovered from the preoperative symptoms without any neurologic deterioration (Fig. 6). Histological examinations confirmed the diagnosis of cystic schwannoma with both Antoni A and Antoni B growth patterns in a fibrillar background (Fig. 7). Verocay bodies were noted, and the tumor cells were reactive to S-100 protein antibody.
The patient was discharged at 1 week after the operation without any complications. Outpatient clinic follow-up for 6 month showed no symptom recurrence and the patient was tolerable. Serial follow-up X-rays at 3 and 6 month after surgery showed no implant-related complications.
Sacral schwannomas are very rare etiology comprising less than 1% to 5% of all spinal schwannomas. Cagli et al.4) reported that only about 50 cases of sacral schwannomas have been presented worldwide. Sacral schwannomas tend to become larger than other spinal tumors because of the relative mobility of the roots and the wide diameter of the spinal canal surrounding it9).
Based on the classification system proposed by Sridhar et al.27), spinal schwannomas can be divided into five types based on their intra- and extraspinal involvement. According to this classification, giant schwannoma is defined as either 1) intraspinal tumor exceeding 2 vertebral segments in length or 2) intraspinal tumor with extraspinal extension exceeding 2.5 cm in length. Spinal schwannoma with erosion into vertebral bodies are defined as "giant invasive tumor" which is type 5 by this classification. Moreover, Klimo et al.16) proposed another classification scheme for sacral nerve sheath tumors based on tumor location; 1) Type 1 confined to the sacrum and may be resected with posterior approach, 2) Type 2 breaching the anterior and posterior osseous margins requiring combined anterior and posterior approach, 3) Type 3 located within the presacral space and an anterior approach is necessary. Our case is a Sridhar Type 5, Klimo Type 1 tumor which is a giant invasive tumor and may be treated with posterior approach alone.
The initial presenting symptoms are mild, non-specific and not until the tumor becomes large enough to displace nerve root peripherally that the patient experience pain or numbness. Axial back pain or radicular pain is the most common symptoms. Delayed presentation is common due to slow growing nature of the tumor and the surrounding anatomic environment is somewhat permissive. Moreover, most of the patients' are young and otherwise healthy and neurological deficits may be minimal despite of large mass size.
The presence of a predominantly cystic schwannoma in the lumbosacral region is rare. These cystic changes are likely attributable to mucinous degeneration, ischemic necrosis, intra-tumor hemorrhage, and the formation of microcysts. Purely cystic tumor is very unusual finding in the spinal schwannoma, although there are a few reports of dominant cystic component in other anatomic locations15,18,21).
The initial differential diagnosis should include relatively large, sacral bone-erosive masses such as abscess, chordoma, metastatic carcinoma, myeloma, giant cell tumor, ABC, chondrosarcoma, osteoblastoma, and schwannoma5). Histopathological examination remains mainstay of diagnosis as clinicoradiographic features can be indistinguishable. Although there are no pathognomic radiographic findings for schwannoma, rim enhancement of the cystic potion of tumor is maybe suggestive of the diagnosis8).
ABC is a benign, tumor-like, highly vascular, locally aggressive and osteolytic lesion of unknown etiology. ABCs are known to have a predilection for the lumbar spine but in some clinical series sacrum was involved more than others30). Characteristic "ballooning" of the posterior column with a thin peripheral rim may be shown on plain radiographs. CT scan reveals multiloculated osteolytic lesions with multiple septated cysts along with pathologic fracture or vertebral body collapse. A thin, well defined rim of low signal intensity in the periphery with multiple internal septation is highly suggestive of ABC in MR imaging. Fluid-fluid levels can be seen but not specific for ABCs. In one study, fluid-fluid levels were present only in half of cases30).
In our case, the initial diagnosis was ABC because of sacral location, typical ballooning with thin peripheral rim in plain film, multiloculated osteolytic lesions with septation on CT scan, pathologic fracture of upper S1 endplate and well defined peripheral rim with multiple internal septation on MR imaging. We did not perform conventional spinal angiography as it is not essential preoperative study for ABC and therefore considered too invasive. Although, usual fluid-fluid level was not present, both radiologist and neurosurgeons strongly suggested ABC as the first differential diagnosis. Cystic schwannoma should be considered as a differential diagnosis option when treating osteolytic sacral cysts suggestive of ABC.
Plain radiographs and CT scans demonstrate the bone destruction and are required to evaluate the spinal stability as most giant schwannomas arising within the spine grow to a large size and result in pedicle erosion, and widening of the neural foramen with occasional destruction of the vertebra bodies. In cases of cystic schwannomas, CT usually shows a non-specific, well defined lesion with low or mixed signal, rarely with areas of cystic necrotic centre7). MRI is helpful in detecting the specific characteristics of the tumor. It provides information about the size, exact location, and invasion of and relationship with other organs. Typical findings of schwannomas in MRI are low signal on T1-weighted and high signal on T2-weighted images7), while degenerated areas and fibrous tumor capsule are the most useful radiological criteria for the cystic types12). Reported cases suggests that giant schwannoma shows heterogeneous T1 and T2 signal and contrast enhancement11,24).
Definitive diagnosis is based on histological examinations. The histologic findings of cystic schwannoma do not seem to differ from classic schwannomas which consists of spindle-shaped cells with pale, eosinophilic cytoplasm arranged in 2 characteristic patterns : Antoni A (compact, hypercellular spindle cells in "palisading" organization) and Antoni B (hypocellular, pleomorphic cells with predominantly myxoid cytoplasm)2,29). Sparse mitotic hyperchromatic nuclei and degenerative changes, such as cyst formation, calcification, with only occasional sites of hemorrhage are the major histopathological characteristics of the ancient forms of schwannomas20). The exact developmental mechanism of cystic degeneration for this type of tumor remains unknown and there is no report in the literature demonstrating a histological change. There are different theories to explain the cystic changes rarely observed in spinal schwannomas. One theory is that cystic areas are secondary to the coalescence of mucinous or microcystic regions in Antoni B tissues of the schwannoma10), which can progress in size over time. Another is that tumor growth lead to insufficient vascular supply resulting in central necrosis and hemorrhage into the tumor with blood resorption causing cyst formation18,25).
Complete excision is recommended for management of giant spinal schwannoma because partial resection has a risk of recurrence14,26). Repeated surgeries are more difficult and dangerous because of unfamiliar sacral anantomy. Complete excision without sacrificing nerve roots is feasible in most of the cases as although schwannomas originate from nerve sheath, only 50% of cases have a direct relation with a nerve roots17,28). The choice surgical approach is dependent upon the degree of sacral involvement and extrapsinal extension. The posterior approach should be used in cases of intraspinal tumors. To achieve total resection, a combined approach should be considered when tumor extends beyond the anterior vertebral body. Our experience suggests when the extraspinal portion is greater than the intraspinal and vertebral body portions and more than one-third of the mass is beyond the anterior vertebral body, a combined approach is preferred. Otherwise, the posterior approach alone should be enough. Surgical treatment of giant sacral cystic schwannomas can be very demanding due to number of reasons; 1) adhesion of the tumor capsule to the surrounding neural structures, 2) fragile tumor capsules, 3) relatively large size of tumor, and 4) increased vascularity in the sacral region. Early identification of the capsule margins without opening of the cyst and sharp dissection may be helpful.
Spinal stabilization is one of the important goals in the treatment of large sacral tumors with bony erosions. Kagaya et al.14) demonstrated that half of the reported cases after subtotal removal of giant cauda equina schwannomas needed spinal reconstruction because of the spinal instability. Sridhar et al.27) recommended lumbosacral stabilization when 25% of the vertebral body is compromised and more recently reported cases have been treated with various stabilization techniques22,26). Instrumented reconstruction should be considered and preoperatively planned depending on the degree of sacral destruction and sacroiliac joint involvement. Furthermore, multilevel laminectomy with significant facet removal for tumor resection may cause spinal deformity or instability. Our experience and other published literatures suggest that giant sacral schwannoma with significant vertebral body invasion, such as our case, require instrumented stablization.
We present a rare case of pure cystic giant schwannoma confined to sacrum mimicking ABC. The surgical treatment is challenging due to the complex anatomy of the sacrum. A successful surgical outcome depends on early diagnosis and complete excision. Schwannoma should be considered in the differential diagnosis of osteolytic sacral cysts.
References
1. Abernathey CD, Onofrio BM, Scheithauer B, Pairolero PC, Shives TC. Surgical management of giant sacral schwannomas. J Neurosurg. 1986; 65:286–295. PMID: 3734878.

2. Albert AF, Kirkman MA, du Plessis D, Sacho R, Cowie R, Tzerakis NG. Giant solitary cystic schwannoma of the cervical spine : a case report. Clin Neurol Neurosurg. 2012; 114:396–398. PMID: 22104695.
3. Bhatia S, Khosla A, Dhir R, Bhatia R, Banerji AK. Giant lumbosacral nerve sheath tumors. Surg Neurol. 1992; 37:118–122. PMID: 1546375.

4. Cagli S, Isik HS, Yildirim U, Akinturk N, Zileli M. Giant sacral schwannomas. J Neurooncol. 2012; 110:105–110. PMID: 22806341.

5. Chandhanayingyong C, Asavamongkolkul A, Lektrakul N, Muangsomboon S. The management of sacral schwannoma : report of four cases and review of literature. Sarcoma. 2008; 2008:845132. PMID: 18779869.
6. Choudry Q, Younis F, Smith RB. Intraosseous schwannoma of D12 thoracic vertebra : diagnosis and surgical management with 5-year follow-up. Eur Spine J. 2007; 16(Suppl 3):283–286. PMID: 17082954.

7. Cury J, Coelho RF, Srougi M. Retroperitoneal schwannoma : case series and literature review. Clinics (Sao Paulo). 2007; 62:359–362. PMID: 17589680.
8. Friedman DP, Tartaglino LM, Flanders AE. Intradural schwannomas of the spine : MR findings with emphasis on contrast-enhancement characteristics. AJR Am J Roentgenol. 1992; 158:1347–1350. PMID: 1590138.

9. Hanakita J, Suwa H, Nagayasu S, Nishi S, Iihara K, Sakaida H. Clinical features of intradural neurinomas in the cauda equina and around the conus medullaris. Neurochirurgia (Stuttg). 1992; 35:145–149. PMID: 1436363.

10. Hayashi Y, Watanabe T, Kita D, Hayashi Y, Takahira M, Hamada J. Orbital cystic schwannoma originating from the frontal nerve. Case Rep Ophthalmol Med. 2012; 2012:604574. PMID: 23320224.

11. Hung CH, Tsai TH, Lieu AS, Lin CL, Lee KS, Hwang SL, et al. Giant invasive schwannoma of cauda equina with minimal neurologic deficit : a case report and literature review. Kaohsiung J Med Sci. 2008; 24:212–217. PMID: 18424359.

12. Inokuchi T, Takiuchi H, Moriwaki Y, Ka T, Takahashi S, Tsutsumi Z, et al. Retroperitoneal ancient schwannoma presenting as an adrenal incidentaloma : CT and MR findings. Magn Reson Imaging. 2006; 24:1389–1393. PMID: 17145411.

13. Jaiswal A, Shetty AP, Rajasekaran S. Giant cystic intradural schwannoma in the lumbosacral region : a case report. J Orthop Surg (Hong Kong). 2008; 16:102–106. PMID: 18453671.

14. Kagaya H, Abe E, Sato K, Shimada Y, Kimura A. Giant cauda equina schwannoma. A case report. Spine (Phila Pa 1976). 2000; 25:268–272. PMID: 10685494.
15. Kececi Y, Gurler T, Gundogan H, Bilkay U, Cagdas A. Benign giant schwannoma located in the upper arm. Ann Plast Surg. 1997; 39:100–102. PMID: 9229103.

16. Klimo P Jr, Rao G, Schmidt RH, Schmidt MH. Nerve sheath tumors involving the sacrum. Case report and classification scheme. Neurosurg Focus. 2003; 15:E12. PMID: 15350043.
17. Kobayashi S, Uchida K, Kokubo Y, Yayama T, Nakajima H, Inukai T, et al. A schwannoma of the S1 dural sleeve was resected while the intact nerve fibers were preserved using a microscope. Report of a case with early MRI findings. Minim Invasive Neurosurg. 2007; 50:120–123. PMID: 17674301.

18. Lam DS, Ng JS, To KF, Abdulah V, Liew CT, Tso MO. Cystic schwannoma of the orbit. Eye (Lond). 1997; 11(Pt 6):798–800. PMID: 9537134.

19. Lesoin F, Krivosic I, Cama A, Jomin M. A giant intrasacral schwannoma revealed by lumbosacral pain. Neurochirurgia (Stuttg). 1984; 27:23–24. PMID: 6700817.

20. Liu YW, Chiu HH, Huang CC, Tu CA. Retroperitoneal schwannoma mimicking a psoas abscess. Clin Gastroenterol Hepatol. 2007; 5:A32. PMID: 17825763.

21. Ogose A, Hotta T, Sato S, Takano R, Higuchi T. Presacral schwannoma with purely cystic form. Spine (Phila Pa 1976). 2001; 26:1817–1819. PMID: 11493858.

22. Ortolan EG, Sola CA, Gruenberg MF, Carballo Vazquez F. Giant sacral schwannoma. A case report. Spine (Phila Pa 1976). 1996; 21:522–526. PMID: 8658260.
23. Parmar H, Patkar D, Gadani S, Shah J. Cystic lumbar nerve sheath tumours : MRI features in five patients. Australas Radiol. 2001; 45:123–127. PMID: 11380354.

24. Parmar HA, Ibrahim M, Castillo M, Mukherji SK. Pictorial essay : diverse imaging features of spinal schwannomas. J Comput Assist Tomogr. 2007; 31:329–334. PMID: 17538274.
25. Pushker N, Meel R, Sharma S, Bajaj MS, Kashyap S, Sen S. Giant orbital schwannoma with fluid-fluid levels. Br J Ophthalmol. 2011; 95:11681180–1181. PMID: 20657016.

26. Saito T, Shimode M, Azuma S, Seichi A. Giant schwannoma of the cauda equina with dural ectasia : a case report. J Orthop Sci. 2004; 9:635–637. PMID: 16228684.
27. Sridhar K, Ramamurthi R, Vasudevan MC, Ramamurthi B. Giant invasive spinal schwannomas : definition and surgical management. J Neurosurg. 2001; 94:210–215. PMID: 11302622.
28. Vikram M, Pande A, Vasudevan MC, Ravi R. Cervical solitary long segment cystic Schwannoma. Br J Neurosurg. 2010; 24:208–210. PMID: 19886817.

29. Wilkinson JS, Mann SA, Robinson CA, Fourney DR. Giant cystic intradural lumbosacral schwannoma: is stabilization necessary? Can J Neurol Sci. 2010; 37:535–538. PMID: 20724268.

30. Zileli M, Isik HS, Ogut FE, Is M, Cagli S, Calli C. Aneurysmal bone cysts of the spine. Eur Spine J. 2013; 22:593–601. PMID: 23053752.

Fig. 1
Simple radiograph shows "ballooning" of osteolytic lesion involving left sacral ala (black arrows) without lumbar spine involvement.
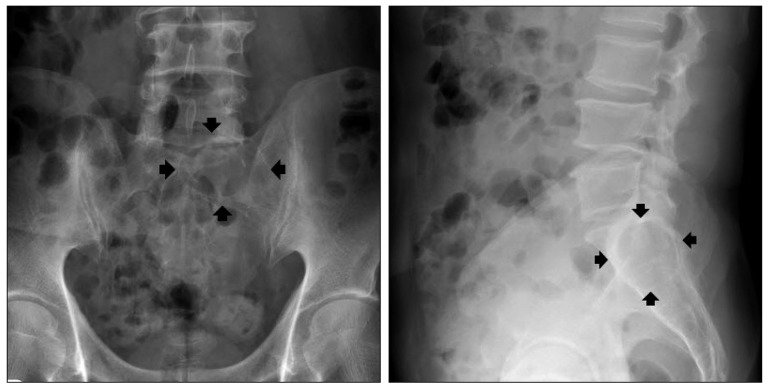
Fig. 2
A : MR imaging shows 5.1×3.4×3.6 cm sized multiloculated cystic mass with bony expansion into left S1 body. Lt. S1 root compression is also seen. The mass is seen as homogenous hypointense signal on T1-weighted image and hyperintense signal on T2-weighted image. B : Multi-septated cystic rim enhancement with gadolinium contrast enhancement is noted but no definite solid portion was found.
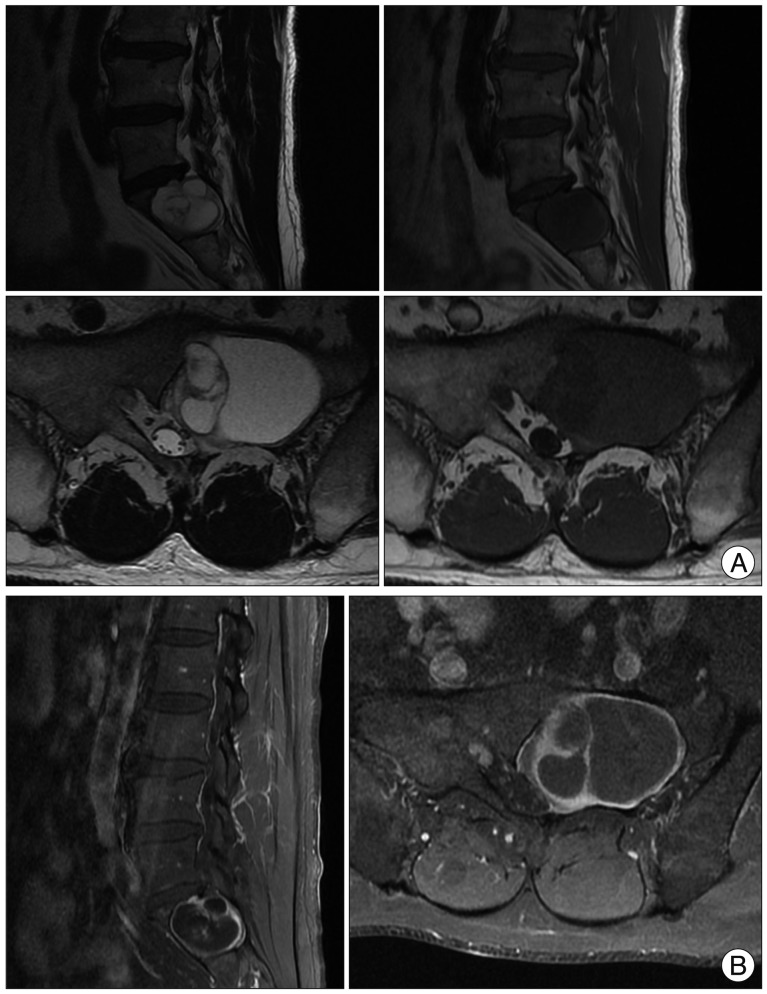
Fig. 3
Axial and sagittal CT scan reveal an intraosseous expensile mass involving left sacrum. Compression of the left S1 nerve root and displacement (arrowhead) of the left sacral nerves below S1 nerve is noted. Subtle pathologic fracture involving upper-endplate of S1 is also seen (arrow).
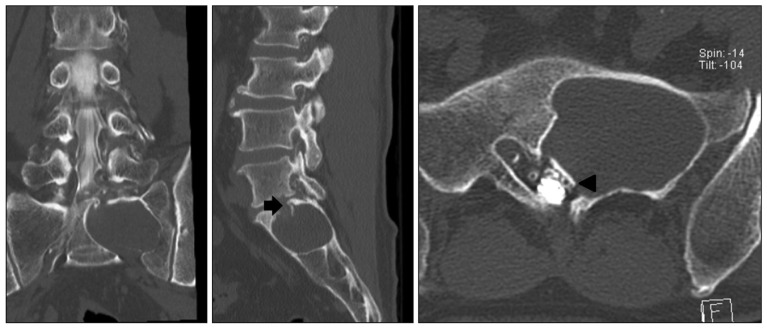
Fig. 4
A : Intraoperative image of before tumor dissection (* : cystic tumor capsule, → : S1 nerve root). B : Intraoperative image of after complete tumor removal. C : Pathologic specimen of tumor cystic capsule.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download