Abstract
Objective
The surgical approach for recurrent pituitary adenoma after trans-sphenoidal approach (TSA) is challenging. We report the outcomes of the endoscopic TSA for recurrent pituitary adenoma after microscopic TSA.
Methods
From February 2010 to February 2013, endoscopic TSA was performed for removal of 30 recurrent pituitary adenomas after microscopic TSA. Twenty-seven (90%) patients had a clinically non-functioning pituitary adenoma. Twenty-four (80%) patients suffered from a visual disturbance related to tumor growth. The clinical features and surgical outcomes were retrospectively analyzed for the ophthalmological, endocrinological, and oncological aspects.
Results
The mean tumor volume was 11.7 cm3, and gross total resection was achieved in 50% of patients. The volumetric analysis based on the postoperative MR showed that the mean extent of resection rates were 90%. Vision was improved in 19 (79%) of 24 patients with visual symptoms, and endocrinological cure was achieved in all of three functioning pituitary adenomas; however, the post-operative follow-up endocrinological examination revealed a new endocrinological deficit in one patient. Two patients required antibiotics management for post-operative meningitis.
Pituitary adenoma is a common primary brain tumor with benign features, and surgical resection continues to be the preferred treatment with the exception of prolactin-secreting tumors20). The nature of the pituitary adenoma itself suggests the possibility of tumor recurrence, regardless of its endocrinological characteristics. Recurrence rate of pituitary adenoma after surgical resection has been reported up to 30%, and regrowth after incomplete tumor removal was reported in up to 75% of cases4).
Recurrent tumors can be managed with observation, medical therapy, radiotherapy, radiosurgery, or revision surgery. Additionally, the combined treatment paradigm is essential in some cases because all treatment modalities have advantages and disadvantages. Surgery for a recurrent lesion is burdened by increased risk of mortality and morbidity, and it often results in incomplete resection compared with initial surgery3,4,7,10,17). Radiosurgery and stereotactic radiotherapy can be recommended as adjuvant treatments to obtain long-term control with low procedure-related morbidity in selective cases. However, the repeated surgical resection is inevitable when a tumor is very large, close to the optic apparatus, or hormone-secreting.
During the last century, pituitary surgery has been developed with various technical modifications and instrumental advances, and the outcome has been generally excellent, with high rates of clinical improvement and endocrinological remission and minimal rates of morbidity and mortality11). The introduction of endoscopy in the trans-sphenoidal approach (TSA) has tremendously advanced the midline skull base surgery. In the literatures, the efficacy and safety of endoscopic TSA in the management of pituitary adenomas have been proven and the complication rates of endoscopic TSA are at least comparable with those of microscopic series17,18,28). However, the reports on endoscopic TSA as a revision surgery have been rarely published5,32).
We retrospectively evaluated the efficacy and safety of the endoscopic TSA for recurrent pituitary adenomas following microscopic TSA.
With approval from Institutional Review Board (No. 1007-066-323), the authors retrospectively reviewed the medical record and imaging data of patients who underwent surgical resection of recurrent pituitary adenomas via endoscopic TSA. A recurrent pituitary adenoma was defined as a newly developed pituitary adenoma without the evidence of residual tumor on magnetic resonance images (MRIs) taken at least 6 months after previous microscopic TSA or a growing residual pituitary adenoma on serial post-operative MRIs. We recommended revision surgery only for recurrent tumors with clear clinical mass effect or endocrinological evidence of hypersecretion.
This study included thirty patients who underwent endoscopic TSA between February 2010 and February 2013 and were then followed up regularly. All patients underwent regular neuro-ophthalmological, endocrinological, and radiological evaluations including uncorrected visual acuity and Goldmann perimetry testing, before and at 1 and 3 months after surgery. The degrees of visual disturbance were analyzed according to the guidelines of the German Ophthalmological Society, which assess bilateral visual acuity and field using the visual impairment scale (VIS). The VIS ranges from 0 (best) to 100 (worst)13).
An early morning basal hormone study was performed in all patients, and the adrenocortical axis was evaluated by a cocktail test or a rapid adrenocorticotropic hormone-stimulating test before and 3 months after surgery1). In cases with a growth hormone-secreting tumor, disease control was determined by the following criteria from the 2010 consensus guidelines : an IGF-I value within normal range for age and gender and a growth hormone (GH) value <0.4 ng/mL after a 75 g oral glucose load or a random GH value <1.0 ng/mL14). Remission of Cushing's disease was defined as adequate suppression of serum cortisol in the morning after administering 1 mg of dexamethasone and normalization of urinary free cortisol excretion within three months after surgery1).
The volumetric analysis, based on the pre- and post-operative MRIs, was performed in all cases to evaluate the extent of tumor removal. The post-operative neuroradiological evaluation consisted of MRIs within 24 hours of the surgery. Using Marosis® Version 5.4 (Infinite, Seoul, Korea), the contour of the tumor, on T1-weighted coronal image with the contrast, was traced using freehand tools and the actual area was measured. The tumor volume was calculated by integrating each tumor area by the slice thickness of the image29). The quantitative degree of tumor resection was expressed as the resection rate, the ratio of removed tumor volume to pre-operative tumor volume. In addition, the degree of cavernous invasion was described using the Knosp classification24).
For analyzing associations between visual outcome and several factors, Mann-Whitney U test was used and statistical significance was accepted at probability values of less than 0.05. These statistical analyses were performed with the aid of SPSS software (version 19.0; SPSS Inc., Chicago, IL, USA).
All procedures were performed using a binaural, endoscopic endonasal trans-sphenoid trans-sellar approach. The trans-sphenoid trans-tubercular approach was performed in one patient. The pedicled nasoseptal flap was prepared when the large defect of diaphragm or the exposure of internal carotid artery (ICA) were expected. The extent of sphenoidotomy was made to visualize the tuberculum and clival recess. The wide panoramic view of endoscope had a critical role to identify the skullbase bony structures. After sphenoidotomy, we always identified the bilateral optic canals and clival ICA protuberances before removing scar tissue on sella, because the scar tissue often precluded the identification of the bilateral cavernous ICA protuberances. The dissection of fibrous tissues on the sella started at the imaginary midline line from the midpoint bilateral optic canals to the midline of clival recess and continued to expose the medial margin of cavernous sinus. The autologous or artificial reconstructive materials used in previous surgery adhered to the dura. Therefore, we drilled circumferentially the margin of previous sellar opening in order to visualize the adhesion site between dura and reconstructive materials and avoid the unintended dural damage. The dissection of reconstruction materials was performed gently and then the wide sellar bony window was created. After a creating dural opening, we attempted to performed extracapsular dissection circumferentially. Internal decompression with piecemeal fashion was performed in cases without a prominent pseudocapsule. The ICAs were reconfirmed with intra-operative Doppler. We tried to remove the intracavernous tumor through the defect of cavernous wall made by the tumor from medial to lateral side to ICA6,23). The direct approach to the lateral component of cavernous sinus was performed only when the tumor completely surrounded the anterior surface of the ICA siphon portion. The tumor in the cavernous sinus was removed under direct view of angled endoscope (30 or 45 degree) in all cases and blind curettage was never used. We have kept the policy that tumors adhering severely to the ICA or pituitary gland were not removed and the blind curettage was not used in order to avoid dangerous conditions. No special reconstruction technique of skull base defect was required in of any patients without intraoperative cerebrospinal fluid (CSF) leakage, however, we combined various techniques such as multi-layers of collagen fleece coated with fibrin sealant, on-lay graft of injectable hydroxyapatite cement, fascia graft, and pedicled nasoseptal flap to achieve multi-layer reconstruction when intraoperative CSF leakage was occurred8,9,19,22,26).
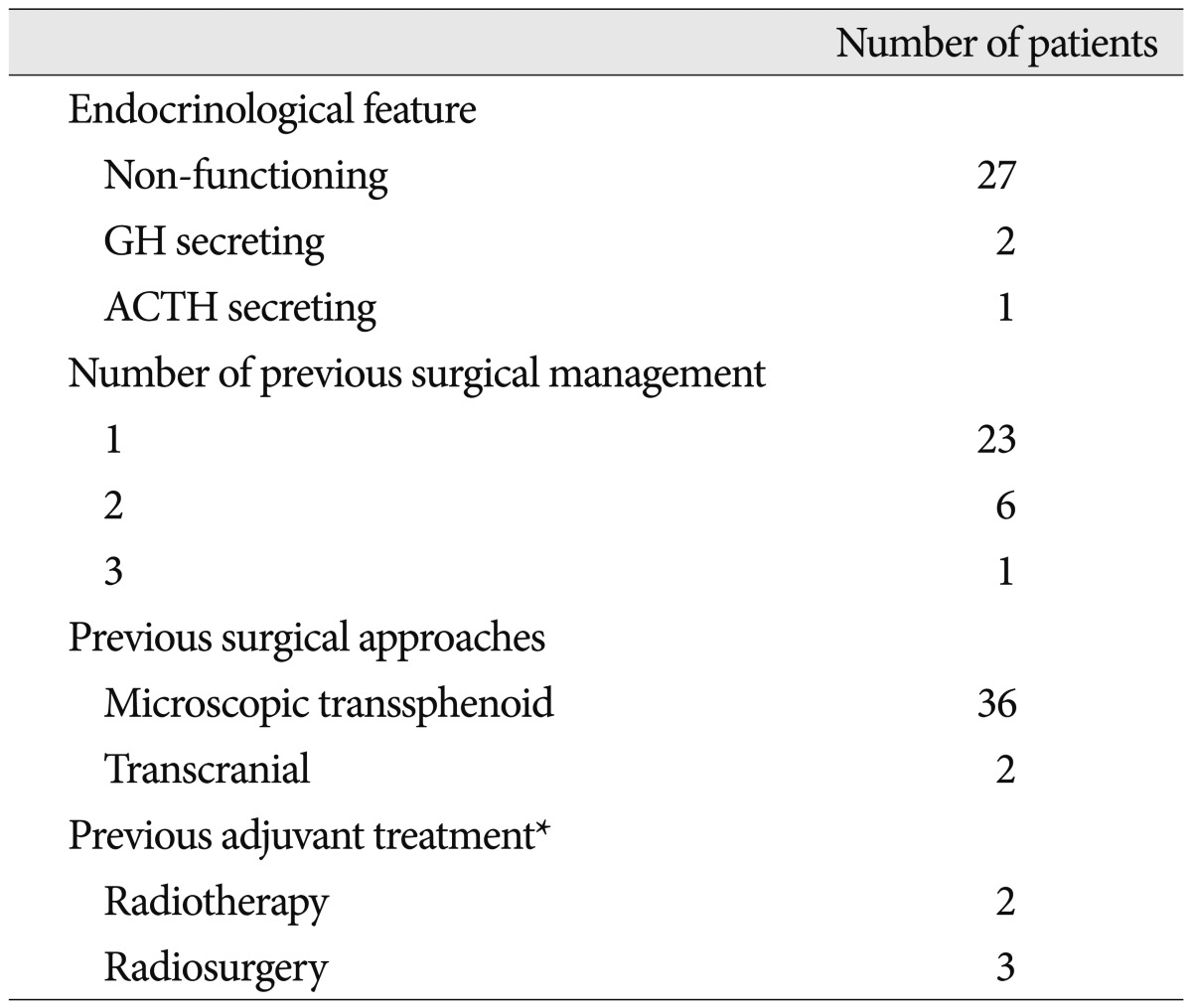
Thirty patients (male : female=9 : 21) with a mean age of 41 years (range, 17-68 years) were evaluated, including six patients with a recurrent pituitary adenoma and twenty-four patients with a residual pituitary adenoma after microscopic TSA. Twenty-seven tumors were regarded as non-functioning pituitary adenomas based on preoperative endocrine status. The functioning pituitary adenomas consisted of one adrenocorticotropic hormone (ACTH)-secreting adenoma and two GH-secreting adenomas. The overall interval between the last surgery and tumor recurrence/regrowth ranged from 1 to 163 months (mean, 64 months). The mean interval between previous surgery and revision endoscopic TSA was 70 months in non-functioning pituitary adenomas. Four of thirty patents received adjuvant radiosurgery or radiotherapy. The pre-operative clinical features are summarized in Table 1.
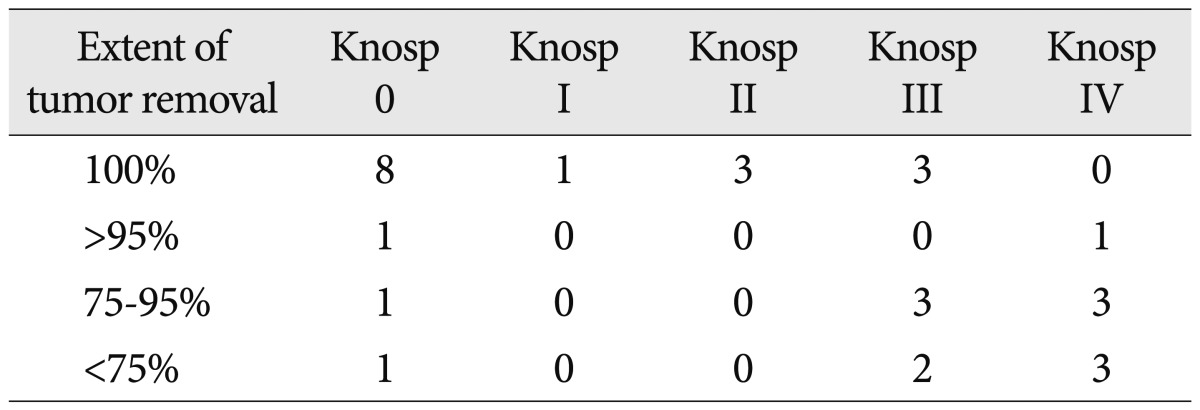
The tumor volume ranged from 0.1 to 41.8 cm3 (mean, 11.4 cm3). All three tumors less than 1 cm3 were functioning pituitary adenomas. Post-operative MRIs demonstrated that tumors were completely resected in 15 (50%) patients. The mean residual tumor volume was 2.9 cm3 (range, 0.3 to 11.7 cm3) and remnant tumors larger than 3 cm3 was observed in 5 patients. The volumetric analysis revealed the extent of tumor removal ranged from 52% to 100% (mean, 90%). Three out of eleven tumors in Knosp grade I was removed subtotally and their volumes were over 15 cm3. Gross total resection rate in Knosp grade I and II were 100%, respectively. Three of eight tumors (38%) in Knosp grade III and none of seven tumors in Knosp grade IV were removed totally. The relationship between the extent of tumor removal and Knosp grade was summarized in Table 2. The cause of subtotal resection was adhesion to the ICA in eleven patients and distorted anatomical structures in four. After the endoscopic TSA, stereotactic radiosurgery (SRS) was performed in all but two patients with residual tumors who refused it.
The mean pre- and post-operative VISs were 43.1 and 26.8, respectively. The visual status of six patients without recent objective or subjective visual deterioration was not changed after the endoscopic TSA, and their mean VIS score was 21.
A newly developed visual disturbance caused by the recurrent tumor was documented in 24 (80%) patients. The mean pre- and post-operative VIS scores in patients with tumor-related visual deterioration were 48 (range, 2-100) and 28 (range, 0-100), respectively. Nineteen (79%) of twenty-four patients showed improved visual status at the postoperative follow-up ophthalmic examination. The postoperative VIS was 0 in five (21%). However, the visual status was unchanged in 4 (17%) of 24 patients. The mean preoperative VIS in patients with the better visual status after surgery was statistically lower than that of those without the benefit in vision (42 versus 79, p=0.044). The preoperative tumor volume, resection rate, and residual tumor volume had no statistical significance in visual improvement.
One patient who suffered from sixth cranial nerve palsy without optic neuropathy before surgery had completely recovered in postoperative 2 months.
The pre-operative endocrinological evaluation revealed panhypopituitarism in 7 of 27 non-functioning pituitary adenomas, GH deficiency in two, and elevated serum prolactin in one. New panhypopituitarism did not develop after surgery in any of seventeen patients with intact endocrinological function. New postoperative hypocortisolism developed in one patient with a previous GH deficit. Transient diabetes insipidus occurred in 4 (17%) of 23 patients without pre-operative panhypopituitarism, and resolved within 3 weeks after surgery in all four cases. Endocrinological cure was achieved in all three patients with functioning pituitary adenomas, including two GH-secreting tumors and one ACTH-secreting tumor.
No death or major morbidity related to surgical procedures was observed. There was no post-operative CSF leakage. However, two patients, in whom intraopearive CSF leakage occurred, required the antibiotic medication for postoperative meningitis and were cured without any sequelae within two weeks. The post-operative pulmonary embolism occurred in one patient with poorly controlled diabetes mellitus and successfully managed with anti-coagulation therapy.
The characteristic bony protuberances and grooves of the skull base bone, secondary changes by aeration of the sphenoid sinus, play the critical roles in TSA as anatomical markers. However, it is difficult to identify and orient the relevant anatomic locations in the revision TSA because of granulation tissue in the sphenoid sinus and loss of the normal bony indentation of the skull base. The appropriate tumor exposure and precise resection in the revision surgery are more difficult and higher complications rates were reported, even when performed by experienced surgeons33). The exact anatomical orientation based on the surrounding landmarks is a key for a success of revision TSA and it could be achieved by the wide surgical exposure or panoramic view in the limited surgical window in endoscopic TSA. The importance of wide sphenoid and sellar exposure had been proved by Mattozo et al.27). Their microscopic revision TSA of the 30 consecutive cases revealed that the suboptimal exposure of the sphenoid keel, the sella, or both was found in 97, 93, and 90%, respectively. They concluded that most pituitary adenoma without cavernous sinus invasion could be totally removed in the revision surgery with a wider exposure. Cavallo et al.5) suggested the wide anterior sphenoidotomy in endoscopic TSA allowed a better dissection of scar tissue in sphenoid sinus and wider opening of sellar floor even in revision surgery following microscopic TSA. Compared to a microscope, the wide and multi-angled view of endoscope offers a better anatomic orientation along the entire surgical route to sella even in revision surgery, although the anatomical features of nasal cavity could be helpful21). Cappabianca and Solari4) also commented that these optical features make it relatively easy to identify the relevant target on the posterior sphenoid surface and the tumor-gland interface, which is even more important in patients who have already undergone the TSA. Owing to wide panoramic view, the complete discrimination of the scar tissues and precise exposure of sellar bony window could be assured easily in our experiences.
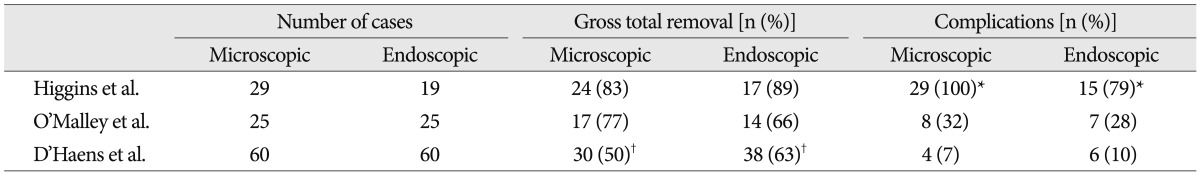
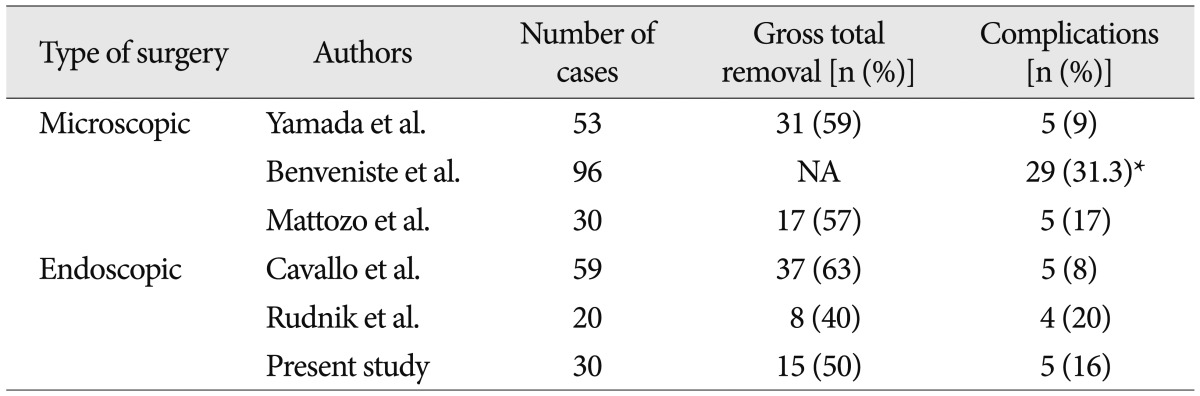
The efficacy of the endoscopic TSA for the management of pituitary tumors has been reported in the literature, with results and complication rates at least comparable with microsurgical series (Table 3)12,17,28). A meta-analysis of eleven published studies revealed no difference in endocrinological remission rate of functioning adenomas (60% versus 66%) and complete tumor removal rate (69% versus 71%) between microscopic and endoscopic TSA. Post-operative diabetes insipidus was less frequent in those having endoscopic surgery (28% versus 15%)16). However, it has not been elucidated well whether the endoscopic TSA could provide a similar or better outcome than microscopic TSA for recurrent pituitary adenoma despite of theoretical benefits. The reported series were a few in number and not the randomized prospective multi-center trials but the retrospective single institution series. Moreover, the extent of tumor and relationship with critical neurovascular structures, which are critical factors for the degree of tumor resection, are too heterogeneous in recurrent pituitary adenomas. In addition, to the best our knowledge, there has not been reported that two surgical techniques were performed by a surgeon. However, the reports in the literatures on revision TSA with single surgical method have showed that endoscopic approach achieved at least comparable outcomes with microscopic approaches (Table 4)3,5,31,33).
Total resection rate of 50% in present study was similar to other reports and the most common cause of subtotal resection was the adhesion to the critical neurovascular structures. We advocated that the main goal of revision surgery in non-functioning pituitary adenoma was the resolution of mass effect with minimization of surgical risk, because the functional recovery was not resulted only from the total removal of tumor as shown in our prognostic factor for visual outcome. From this view, it was inadequate that the extent of tumor removal was categorized just as total or subtotal resection, because the small residual tumors with effective decompression had a completely different clinical impact compared with the relatively large residual tumors. The volumetric analysis on the extent of tumor removal, which over 75% of resection rate was found in 80% of patients, indicated the endoscopic TSA was an effective and promising surgical technique.
There are several treatment options for small residual pituitary adenoma, including observation, conventional radiotherapy and SRS. The observation should be considered for the asymptomatic residual tumors, considering the indolent natural history of pituitary adenomas, although radiotherapy and SRS have shown excellent tumor control15). And it brought our follow-up period before revision TSA was as long as 70 months. However, we recommended the adjuvant SRS for the residual tumors after revision endoscopic TSA, because the growth potential of tumors was documented in the follow-up period before revision surgery.
There were various complications after TSA, regardless of optical instruments. A meta-analysis revealed the endoscopic TSA was associated with higher incidence of vascular complications associated with a more sphenoid and sellar exposure2). A recent paper on 555 consecutive series of endoscopic TSA for pituitary adenoma reported 19% of complication rate including 2 ICA injuries30). It has been clear that the complication rate of a second TSA is clearly higher than that of primary surgery, even by experienced surgeons and overall complications rates ranged from 9.4% to 31.3%3,5,25,31,33). Considering the previous reports, our results showed the complication rate of endoscopic approach in revision TSA was at least comparable to those of the major microsurgical TSA and primary endoscopic TSA series3,7,10,17,18,33).
References
1. Alwani RA, de Herder WW, de Jong FH, Lamberts SW, van der Lely AJ, Feelders RA. Rapid decrease in adrenal responsiveness to ACTH stimulation after successful pituitary surgery in patients with Cushing's disease. Clin Endocrinol (Oxf). 2011; 75:602–607. PMID: 21623858.

2. Ammirati M, Wei L, Ciric I. Short-term outcome of endoscopic versus microscopic pituitary adenoma surgery : a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2013; 84:843–849. PMID: 23243265.

3. Benveniste RJ, King WA, Walsh J, Lee JS, Delman BN, Post KD. Repeated transsphenoidal surgery to treat recurrent or residual pituitary adenoma. J Neurosurg. 2005; 102:1004–1012. PMID: 16028758.

4. Cappabianca P, Solari D. The endoscopic endonasal approach for the treatment of recurrent or residual pituitary adenomas : widening what to see expands what to do? World Neurosurg. 2012; 77:455–456. PMID: 22120280.

5. Cavallo LM, Solari D, Tasiou A, Esposito F, de Angelis M, D'Enza AI, et al. Endoscopic Endonasal Transsphenoidal Removal of Recurrent and Regrowing Pituitary Adenomas : Experience on a 59-Patient Series. World Neurosurg. 2013; 80:342–350. PMID: 23046913.

6. Ceylan S, Koc K, Anik I. Endoscopic endonasal transsphenoidal approach for pituitary adenomas invading the cavernous sinus. J Neurosurg. 2010; 112:99–107. PMID: 19480546.

7. Chang EF, Sughrue ME, Zada G, Wilson CB, Blevins LS Jr, Kunwar S. Long term outcome following repeat transsphenoidal surgery for recurrent endocrine-inactive pituitary adenomas. Pituitary. 2010; 13:223–229. PMID: 20217484.

8. Cho JM, Ahn JY, Chang JH, Kim SH. Prevention of cerebrospinal fluid rhinorrhea after transsphenoidal surgery by collagen fleece coated with fibrin sealant without autologous tissue graft or postoperative lumbar drainage. Neurosurgery. 2011; 68:130–136. discussion 136-137. PMID: 21206312.

9. Chung SB, Nam DH, Park K, Kim JH, Kong DS. Injectable hydroxyapatite cement patch as an on-lay graft for the sellar reconstructions following endoscopic endonasal approach. Acta Neurochir (Wien). 2012; 154:659–664. discussion 664. PMID: 22350441.

10. Ciric I, Ragin A, Baumgartner C, Pierce D. Complications of transsphenoidal surgery : results of a national survey, review of the literature, and personal experience. Neurosurgery. 1997; 40:225–236. discussion 236-227. PMID: 9007854.

11. Couldwell WT, Weiss MH, Rabb C, Liu JK, Apfelbaum RI, Fukushima T. Variations on the standard transsphenoidal approach to the sellar region, with emphasis on the extended approaches and parasellar approaches : surgical experience in 105 cases. Neurosurgery. 2004; 55:539–547. discussion 547-550. PMID: 15335421.
12. D'Haens J, Van Rompaey K, Stadnik T, Haentjens P, Poppe K, Velkeniers B. Fully endoscopic transsphenoidal surgery for functioning pituitary adenomas : a retrospective comparison with traditional transsphenoidal microsurgery in the same institution. Surg Neurol. 2009; 72:336–340. PMID: 19604551.
13. Fahlbusch R, Schott W. Pterional surgery of meningiomas of the tuberculum sellae and planum sphenoidale : surgical results with special consideration of ophthalmological and endocrinological outcomes. J Neurosurg. 2002; 96:235–243. PMID: 11838796.

14. Giustina A, Chanson P, Bronstein MD, Klibanski A, Lamberts S, Casanueva FF, et al. A consensus on criteria for cure of acromegaly. J Clin Endocrinol Metab. 2010; 95:3141–3148. PMID: 20410227.

15. Gopalan R, Schlesinger D, Vance ML, Laws E, Sheehan J. Long-term outcomes after Gamma Knife radiosurgery for patients with a nonfunctioning pituitary adenoma. Neurosurgery. 2011; 69:284–293. PMID: 21792138.

16. Goudakos JK, Markou KD, Georgalas C. Endoscopic versus microscopic trans-sphenoidal pituitary surgery : a systematic review and meta-analysis. Clin Otolaryngol. 2011; 36:212–220. PMID: 21752205.

17. Higgins TS, Courtemanche C, Karakla D, Strasnick B, Singh RV, Koen JL, et al. Analysis of transnasal endoscopic versus transseptal microscopic approach for excision of pituitary tumors. Am J Rhinol. 2008; 22:649–652. PMID: 19178807.

18. Kabil MS, Eby JB, Shahinian HK. Fully endoscopic endonasal vs. transseptal transsphenoidal pituitary surgery. Minim Invasive Neurosurg. 2005; 48:348–354. PMID: 16432784.

19. Kassam A, Carrau RL, Snyderman CH, Gardner P, Mintz A. Evolution of reconstructive techniques following endoscopic expanded endonasal approaches. Neurosurg Focus. 2005; 19:E8. PMID: 16078822.

20. Kim EY, Park HS, Kim JJ, Han HS, Nam MS, Kim YS, et al. Endoscopic transsphenoidal approach through a widened nasal cavity for pituitary lesions. J Clin Neurosci. 2001; 8:437–441. PMID: 11535013.
21. Kim YH, Kim JE, Kim MJ, Cho JH. New landmark for the endoscopic endonasal transsphenoidal approach of pituitary surgery. J Korean Neurosurg Soc. 2013; 53:218–222. PMID: 23826477.

22. Kitano M, Taneda M. Subdural patch graft technique for watertight closure of large dural defects in extended transsphenoidal surgery. Neurosurgery. 2004; 54:653–660. discussion 660-661. PMID: 15028140.

23. Kitano M, Taneda M, Shimono T, Nakao Y. Extended transsphenoidal approach for surgical management of pituitary adenomas invading the cavernous sinus. J Neurosurg. 2008; 108:26–36. PMID: 18173307.

24. Knosp E, Steiner E, Kitz K, Matula C. Pituitary adenomas with invasion of the cavernous sinus space : a magnetic resonance imaging classification compared with surgical findings. Neurosurgery. 1993; 33:610–617. discussion 617-618. PMID: 8232800.

25. Long H, Beauregard H, Somma M, Comtois R, Serri O, Hardy J. Surgical outcome after repeated transsphenoidal surgery in acromegaly. J Neurosurg. 1996; 85:239–247. PMID: 8755752.

26. Luginbuhl AJ, Campbell PG, Evans J, Rosen M. Endoscopic repair of high-flow cranial base defects using a bilayer button. Laryngoscope. 2010; 120:876–880. PMID: 20422679.

27. Mattozo CA, Dusick JR, Esposito F, Mora H, Cohan P, Malkasian D, et al. Suboptimal sphenoid and sellar exposure : a consistent finding in patients treated with repeat transsphenoidal surgery for residual endocrine-inactive macroadenomas. Neurosurgery. 2006; 58:857–865. discussion 857-865. PMID: 16639319.

28. O'Malley BW Jr, Grady MS, Gabel BC, Cohen MA, Heuer GG, Pisapia J, et al. Comparison of endoscopic and microscopic removal of pituitary adenomas : single-surgeon experience and the learning curve. Neurosurg Focus. 2008; 25:E10. PMID: 19035697.
29. Oya S, Kim SH, Sade B, Lee JH. The natural history of intracranial meningiomas. J Neurosurg. 2011; 114:1250–1256. PMID: 21250802.

30. Paluzzi A, Fernandez-Miranda JC, Tonya Stefko S, Challinor S, Snyderman CH, Gardner PA. Endoscopic endonasal approach for pituitary adenomas : a series of 555 patients. Pituitary. 2013; 8. 02. [Epub ahead of print].
31. Rudnik A, Zawadzki T, Galuszka-Ignasiak B, Bazowski P, Duda I, Wojtacha M, et al. Endoscopic transsphenoidal treatment in recurrent and residual pituitary adenomas--first experience. Minim Invasive Neurosurg. 2006; 49:10–14. PMID: 16547875.

32. Wagenmakers MA, Netea-Maier RT, van Lindert EJ, Timmers HJ, Grotenhuis JA, Hermus AR. Repeated transsphenoidal pituitary surgery (TS) via the endoscopic technique : a good therapeutic option for recurrent or persistent Cushing's disease (CD). Clin Endocrinol (Oxf). 2009; 70:274–280. PMID: 18616702.

33. Yamada S, Fukuhara N, Oyama K, Takeshita A, Takeuchi Y. Repeat transsphenoidal surgery for the treatment of remaining or recurring pituitary tumors in acromegaly. Neurosurgery. 2010; 67:949–956. PMID: 20881560.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download