Abstract
Objective
MR perfusion and single photon emission computerized tomography (SPECT) are well known imaging studies to evaluate hemodynamic change between prior to and following superficial temporal artery (STA)-middle cerebral artery (MCA) anastomosis in moyamoya disease. But their side effects and invasiveness make discomfort to patients. We evaluated the ivy sign on MR fluid attenuated inversion recovery (FLAIR) images in adult patients with moyamoya disease and compared it with result of SPECT and MR perfusion images.
Methods
We enrolled twelve patients (thirteen cases) who were diagnosed with moyamoya disease and underwent STA-MCA anastomosis at our medical institution during a period ranging from September of 2010 to December of 2012. The presence of the ivy sign on MR FLAIR images was classified as Negative (0), Minimal (1), and Positive (2). Regions were classified into four territories: the anterior cerebral artery (ACA), the anterior MCA, the posterior MCA and the posterior cerebral artery.
Results
Ivy signs on preoperative and postoperative MR FLAIR were improved (8 and 4 in the ACA regions, 13 and 4 in the anterior MCA regions and 19 and 9 in the posterior MCA regions). Like this result, the cerebrovascular reserve (CVR) on SPECT was significantly increased in the sum of CVR in same regions after STA-MCA anastomosis.
Conclusion
After STA-MCA anastomosis, ivy signs were decreased in the cerebral hemisphere. As compared with conventional diagnostic modalities such as SPECT and MR perfusion images, the ivy sign on MR FLAIR is considered as a useful indicator in detecting brain hemodynamic changes between preoperatively and postoperatively in adult moyamoya patients.
The ivy sign might be suggestive of diffuse leptomeningeal collaterals found on post-contrast T1-weighted MR images in patients with moyamoya disease11,19). It has also been defined as a continuous linear leptomeningeal high-signal intensity along the cortical sulci and subarachnoid spaces on MR fluid-attenuated inversion recovery (FLAIR) images13). Its proliferation is suggestive of a retrograde slow vascular flow that is highly correlated with the existence of a decreased cerebrovascular reserve (CVR)2,4,5,10,18). Accordingly, recent studies have examined the usefulness of MR FLAIR images as a follow-up study after superficial temporal artery (STA)-middle cerebral artery (MCA) anastomosis in patients with moyamoya disease6,9). Kawashima et al.9) reported that the ivy sign on MR FLAIR images was a useful indicator in detecting brain hemodynamic changes at a follow-up in patients with moyamoya disease who underwent STA-MCA anastomosis. But, most of these studies have been conducted in younger patients with a mean age of 20-29 years. Considering the age distribution, the higher incidence of moyamoya disease was observed in adults 45 to 49 years of age and the second in children 5 to 9 years of age, their reliability would be questionable on aspect of age1).
Given the above background, we conducted this study to examine the usefulness of the ivy sign on MR FLAIR images as compared with single photon emission computerized tomography (SPECT) and MR perfusion studies in detecting brain hemodynamic changes between preoperatively and postoperatively in adult patients (above 20 years) with moyamoya disease3,12,17).
From Sep 2010 to Dec 2012, twelve adult patients with moyamoya disease visited our medical institution. In these patients (average age, 45 years; range, 23-64 years), moyamoya disease was incidentally found on a regular check-up without notable symptoms or it was detected on examinations for transient ischemic attack (TIA) or cerebral infarction. The Suzuki grade of moyamoya disease was analyzed and then compared with the mean ivy score. Twelve patients and thirteen cases (one having surgery at both hemispheres) underwent STA-MCA anastomosis. Our patients had a mean age of 45 years and they underwent STA-MCA anastomosis on preoperative and postoperative MR FLAIR images.
In each patient, such imaging studies as a MRI, a CT angiography, and a SPECT were performed both preoperatively and postoperatively. The MR images included perfusion and FLAIR ones. A board-certified specialist in radiology reviewed the images and rated the ivy sign scores.
MR perfusion imaging represents a form of functional imaging that assesses alterations in blood flow with additional information on metabolism and regional measures of a specific tracer. This technique can be used to find salvageable ischemic brain tissue which represents the "schemic penumbra". Patients identified with a larger penumbra are more likely to benefit from recanalization therapies making it crucial to properly recognize them23). MR perfusion image was performed on 1.5 and 3T machine (Magnetom Skyra, Siemens, Germany) with spin echo-planar imaging sequences by using the following parameters : repetition time (TR)/echo time (TE), 1950/30 ms; flip angle, 90°; matrix, 128×128; field of view 220×220; section thickness 5 mm; and intersection gap, 1 mm in all patients. Sixty sections and 25 images per section in all patients were obtained before, during and after the administration of contrast agent. Contrast agent 0.2 mmol gadopentetate dimeglumine (Multihense, Patheon, Italy) per kilogram of body weight at a rate of 3 mL/sec was injected by using an MR imaging-compatible power injector (Spectrics; Medrad, Pittsburgh, PA, USA). This was followed by a 20 mL bolus of saline administered at the same injection rate. Perfusion maps of CBV were generated after eliminating the effect of contrast agent recirculation by using gamma-variate curve fitting. We utilized software program (Numaris/4, version syngo MR D13) for evaluation of CBV. We selected region of interest in PLIC and MC using MRIcro software program.
MR FLAIR was also performed on a 3T machine (Magnetom Skyra, Siemens, Germany) with spin echo-planar imaging sequences. Protocol imaging included axial FLAIR sequence (TR : 9000 ms; TE : 95 ms; field of view 199×220; section thickness 5 mm; flip angle, 90°; matrix size : 512×278).
In studies of cerebral vascular reserve by SPECT with the use of N-isopropyl-4-iodoamphetamine (123I-IMP), it is necessary to measure cerebral blood flow at rest and after acetazolamide (ACZ) challenge. The SPECT scanner E-cam (Siemens, Germany) and image processor E-soft (Siemens) were used. The collimator used was a low-energy, ultra-high resolution, fan-beam collimator (83 cps/MBq). Conditions for acquisition were as follows : 5-min acquisition (7.5 s/step, 3°/step, continuous), matrix of 128×128 (3.56 mm/pixel), and radius of rotation of 13 cm. A filtered backprojection method using a ramp filter was utilized to reconstruct images from 5-min data. As a 3D post-filter, a low-pass filter (cutoff 0.4 cycles/cm; order 5.0) was used. Two SPECT images were summated to obtain a slice thickness of 2.4 mm.
The corticosubcortical area of cerebral hemisphere was divided into the following four regions on each hemisphere : the anterior cerebral artery (ACA), the anterior half of the middle cerebral artery (ant-MCA), the posterior half of the MCA (post-MCA), and the posterior cerebral artery (PCA)16) (Fig. 1). The central sulcus is a landmark that separates between the ant-MCA regions and the post-MCA ones. We compared both FLAIR images obtained preoperatively and postoperatively based on the ivy sign scores, for which we used a 3-grade system : Grade 0 (negative; no ivy sign), Grade 1 (minimal; the ivy sign is defined as minimal or equivocal high signal intensity) and Grade 2 (positive; the ivy sign is obviously linear and punctate high signal intensity).
The SPECT results were demonstrated using 20 levels of color ranging from black to white. We give the score from 0 to 100 points, it means that each color was given 5 points. This scoring system was possible because the 20 levels of color spectrum is exactly specified in the SPECT image and this result was confirmed the radiologist. When there was a sufficient cerebral perfusion, it was shown as red colors with an intensity of 60 to 80. We checked the CVR before and after administration of acetazolamide. We applied CVR which results of post-administration of acetazolamide to this study because that it is significantly associated with an increased risk of stroke recurrence in patients with symptomatic cerebral infarction8).
We analyzed the relationship between the ivy sign on MR FLAIR images and CVR on SPECT ones and then compared them between prior to and following the STA-MCA anastomosis.
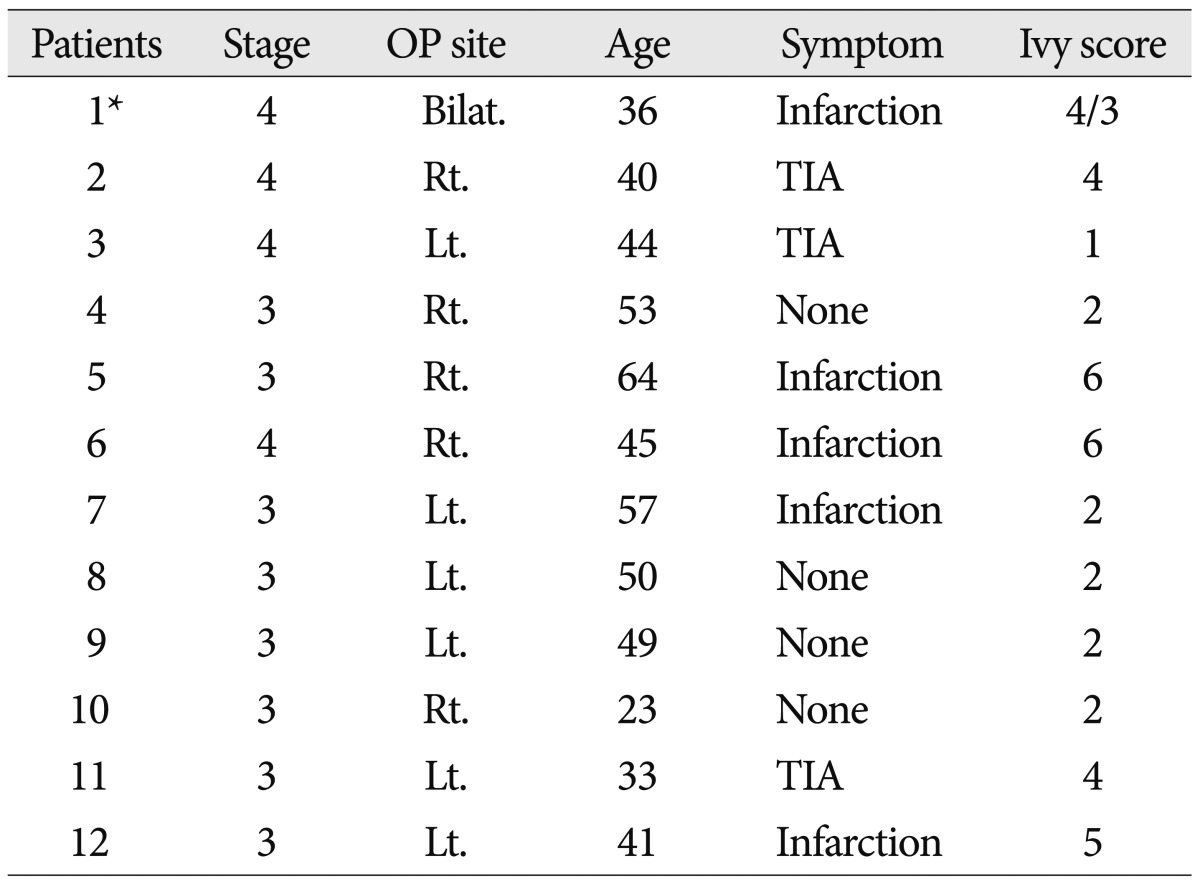
Thirteen cases of twelve patients, one patient to undergo surgery, bilateral hemispheres, were diagnosed with moyamoya disease on angiography and they underwent STA-MCA anastomosis (Table 1). With that as a result of the sum of CVR in all patients, post-contrast image, on the SPECT images was 3035 preoperatively and 3480 postoperatively (p<0.05), the surgery was successful in all patients. Among these patients, one patient got the surgery bilateral STA-MCA anastomosis (Fig. 2) and checked the MR FLAIR and SPECT (Fig. 3).
In order to refine the preoperative and postoperative results, the cerebral hemisphere was divided into four regions : the ACA, ant-MCA, post-MCA, and PCA. Thus, 4 regions of 13 cases, a total of 52 regions were selected. We compared changes in the sum of CVR between preoperatively and postoperatively in each 4 regions. We aimed to proof of the ivy sign could be another marker of hemodynamic change, so the analysis of preoperative and postoperative change of ivy sign is the most important topic in this reports. But ivy sign was not observed on whole region of hemisphere, so we selected the slice of MR FLAIR images which demonstrated the typical ivy signs and compared that with CVR on the same slice of SPECT images. This showed that CVR was increased from 745 to 815 in the ACA region (p<0.05), from 720 to 885 (p<0.05) in the ant-MCA region, and from 710 to 895 (p<0.05) in the post-MCA region. These results indicate that there was a significant increase in the sum of CVR in all of these three regions. In the PCA region, however, it was increased from 860 to 885 but this difference did not reach a statistical significance.
To evaluate the usefulness of ivy sign at a follow-up, we compared ivy sign changes on MR FLAIR images between preoperatively and postoperatively with CVR changes on SPECT images between prior to and following the STA-MCA anastomosis7,19).
All of the patients who showed the minimal or positive ivy sign were identified in 18 of 24 hemispheres (75%). Preoperative and postoperative MR FLAIR images were taken at 46.7 days on average (range, 8-99 days) and at 196.6 days (range, 33-368 days), respectively. The sum of ivy score was 43 preoperatively and 20 postoperatively. This difference reached a statistical significance (p<0.05). Minimal or positive ivy signs were found in 8 ACA regions, 13 ant-MCA regions, 19 post-MCA regions and 3 PCA regions. Of these regions, the ivy signs were predominantly recorded from the MCA territory; their prevalence was the highest in the post-MCA region. By the corticosubcortical area of cerebral hemisphere after STA-MCA anastomosis, ivy score was 8 and 4 in the ACA regions, 13 and 4 in the ant-MCA regions and 19 and 9 in the post-MCA regions. All values were preoperative and postoperative ones in the corresponding order. All of these differences reached a statistical significance (p<0.05). In the PCA regions, however, there was no significant difference in the sum of ivy score between preoperatively (3) and postoperatively (3). In particular, the sum of ivy score was significantly higher in the post-MCA region as compared with other regions and the degree of the reduction in the sum of ivy score was also the highest in the post-MCA region. We compared these results with CVR scores between preoperatively and postoperatively on SPECT images and checked these correlation outcomes reached a statistical significance (p<0.05) (Table 2).
We compared ivy sign changes on MR FLAIR images with MR perfusion images between prior to and following the STA-MCA anastomosis. We selected the same number of the slice of MR perfusion images with the slice of MR flair image, same methods as compared MR FLAIR with SPECT, which demonstrated the typical ivy signs. We compared on MR perfusion images, the degree of change of mean transit time (MTT) was higher in the regions with positive or minimal ivy sign as compared with those with negative one. After STA-MCA anastomosis, it was also demonstrated that the hemodynamic profile was improved in the regions both SPECT (Fig. 4) and perfusion MRI images where the ivy sign was missing or decreased (Fig. 5).
We divided into three groups on patients clinical states; asymptomatic groups, TIA and complete stroke group and checked the change of ivy score by clinical symptoms. The mean preoperative ivy score was 2 points in the asymptomatic group, 3 points in the TIA group and 5.2 points in the complete stroke group, thus indicating that higher ivy score was noticed on the patients with more severe clinical symptoms.
To date, numerous studies have evaluated brain hemodynamic changes. Since brain hemodynamic changes were first measured based on CO2 reactivity in a published study, the SPECT has been regarded as a standard diagnostic modality where the CVR is measured based on the iatrogenic metabolic effect by using an intravenous administration of acetazolamide20,21,23). In addition, the results of MTT on MR perfusion are also useful to determine the hemodynamic change in moyamoya patients12,13). Ohta et al.19) first reported that the ivy sign is a diffuse leptomeningeal enhancement found on T1-weighted MR images of patients with moyamoya disease in childhood. Based on published studies supporting the applicability of ivy sign on T1-weighted MR images in measuring brain hemodynamic changes have been conducted up to present11). But, there are some reports that indicated the side effects occur during the SPECT or MR perfusion, T1-weighted MRI including discomfort to patients. According to Settakis et al.22), side effects such as headache and discomfort may occur as a result of the activation of ACZ. In patients with a low hemodynamic state, there is also a possibility that the CBF might be further reduced with the administration of ACZ because of the vascular steal phenomenon in the adjacent area where the CVR is relatively preserved. Special attention should therefore be paid to the administration of ACZ in patients with severe ischemic symptoms. MR perfusion and T1-weighted MRI also requires administration of contrast agent which eventually require the patient should be evaluated via an intravenous route in a fasting state, so patients should keep the empty stomach for several hours.
According to recent studies, the ivy sign on MR FLAIR images showed a new diagnostic value in detecting brain hemodynamic changes10,13). Several studies had shown that the ivy sign is also seen on MR FALIR images and it is suggestive of brain hemodynamic status in patients with moyamoya disease. We assume that MR FLAIR would be more useful in performing a follow-up evaluation as compared with SPECT or perfusion MRI, T1-weighted MRI study because it causes less discomfort to patients with moyamoya disease15,22). The MR FLAIR study is advantageous in that it can be easily performed in a clinical setting. As compared with SPECT or MR perfusion, T1-weighted MRI study, the MR FLAIR imaging is more advantageous in that it takes less than ten minutes, causes a less side effects, do not need to feel hunger and is a non-invasive modality.
Based on the published studies about the usefulness of the ivy sign on MR FLAIR images in evaluating brain hemodynamic changes, further studies have been conducted to examine whether it is also a useful indicator at a follow-up in patients who underwent STA-MCA anastomosis. In 2010, Kawashima et al.9) used the ivy sign on MR FLAIR images for a follow-up study of patients with moyamoya disease who underwent STA-MCA anastomosis, thus reporting that the ivy sign was a useful indicator in evaluating the preoperative and postoperative hemodynamic status of brain. Ideguchi et al.6) reported that the ivy sign is a non-invasive indicator in detecting brain hemodynamic changes through a comparison between SPECT and MR FLAIR study. However, it should be noted that the above studies enrolled relatively younger patients; Kawashima et al. enrolled patients with a mean age of 25 years and Ideguchi et al. did those with a mean age of 24 years. The detection rate of moyamoya disease per year was 0.94 patients per 100000 people, and prevalence was 10.5 patients per 100000 people. The incidence of ischemia concerned with the disease was 0.53 patients per 100000 people-years and hemorrhage was 0.2 patients per 100000 people-years. And, considering the age distribution, the highest incidence of moyamoya disease was observed in adults 45 to 49 years of age and the second in children 5 to 9 years of age, their reliability would be questionable on aspect of age, the above two studies have the limitation of subject selection in appropriate age1). We performed MR FLAIR study to evaluate the diagnostic value of the ivy sign in adult patients (average age, 45 years; range, 23-64 years). Thus, our results support that it can be considered as a useful indicator for adult patients with moyamoya disease.
We underwent STA-MCA anastomosis in thirteen cases of twelve patients (one patient having surgery on bilateral hemispheres) who were diagnosed with moyamoya disease on angiography. All of the surgery was successful as a result of the sum of CVR on the SPECT images was 3035 preoperatively and 3480 postoperatively (p<0.05).
Based on the reports by Ideguchi et al.6), we compared the ivy signs between preoperatively and postoperatively in each region. Our results showed that it was no significant different in hemodynamic state evaluation between MR FLAIR images and SPECT ones, and based on these results, we conclude that the ivy sign on MR FLAIR images is a useful indicator in detecting brain hemodynamic changes between preoperatively and postoperatively as much as CVR on SPECT ones. To assess the accuracy of MR FLAIR, we separated the results by four regions. Of four areas (ACA, ant-MCA, post-MCA, PCA), results were significant changes in the ivy sign on MR FLAIR images and the CVR on SPECT ones postoperatively as compared with preoperatively in both the ACA and MCA territories and the degree of changes in both indicators was the highest in the MCA territory (p<0.05). But, there were no significant differences in two indicators between preoperatively and postoperatively in the PCA territory (Table 2). These findings can be explained based on the relative preservation of posterior circulation by PCA in patients with moyamoya disease, as reported by Fujiwara et al.23).
Compared to other reports, although it is not absolute comparison, our reports showed that 75% of regions of cerebral hemispheres were positive for the ivy sign; it was higher than 64.7% reported by Kawashima et al. Our report was consistent the report by Mori et al.14,22), ivy sign was a significant correlation with the severity of symptoms. The sum of the ivy scores was 5.2 points in the complete stroke group, 3 points in the TIA group and 2 points in the asymptomatic group. These results are different from other studies indicating that the ivy sign on MR FLAIR is also a useful indicator in detecting brain hemodynamic changes between preoperatively and postoperatively in adult patients (above 20 years) with moyamoya disease.
Limitation of the current study includes a small number of patients (n=13). Also, MR FLAIR was not performed at constant tesla, some patients have taken MRI at 1.5 tesla and others at 3 tesla. Further studies are warranted to compare the ivy signs between preoperatively and postoperatively in patients presenting with clinical symptoms. This would contribute to identifying more significant results of the ivy sign. If there is a significant correlation between the ivy sign and the symptoms, this would be useful to evaluate their prognosis.
The ivy sign on FLAIR images was present in the regions where both the cerebral perfusion and the CVR were decreased. After STA-MCA anastomosis, the ivy sign was disappeared or its intensity was decreased in the hemisphere. As compared with conventional diagnostic modalities such as SPECT or MR perfusion, the ivy sign is considered as a useful indicator in detecting brain hemodynamic changes between preoperatively and postoperatively. Our results also show that it is also a useful indicator in detecting brain hemodynamic changes in adult patients. Furthermore, the degree of ivy sign was higher in patients with a greater severity of symptoms. Further studies are warranted to examine whether the ivy sign is also useful in identifying the improvement of hemodynamic status and assessing the effectiveness of STA-MCA anastomosis in adult patients with moyamoya disease.
References
1. Baba T, Houkin K, Kuroda S. Novel epidemiological features of moyamoya disease. J Neurol Neurosurg Psychiatry. 2008; 79:900–904. PMID: 18077479.

2. Cosnard G, Duprez T, Grandin C, Smith AM, Munier T, Peeters A. Fast FLAIR sequence for detecting, major vascular abnormalities during the hyperacute phase of stroke : a comparison with MR angiography. Neuroradiology. 1999; 41:342–346. PMID: 10379591.

3. Fujimura M, Mugikura S, Shimizu H, Tominaga T. Diagnostic value of perfusion-weighted MRI for evaluating postoperative alteration of cerebral hemodynamics following STA-MCA anastomosis in patients with moyamoya disease. No Shinkei Geka. 2006; 34:801–809. PMID: 16910493.
4. Fujiwara H, Momoshima S, Kuribayashi S. Leptomeningeal high signal intensity (ivy sign) on fluid-attenuated inversion-recovery (FLAIR) MR images in moyamoya disease. Eur J Radiol. 2005; 55:224–230. PMID: 16036151.

5. Gauvrit JY, Leclerc X, Girot M, Cordonnier C, Sotoares G, Henon H, et al. Fluid-attenuated inversion recovery (FLAIR) sequences for the assessment of acute stroke : inter observer and intertechnique reproducibility. J Neurol. 2006; 253:631–635. PMID: 16362529.

6. Ideguchi R, Morikawa M, Enokizono M, Ogawa Y, Nagata I, Uetani M. Ivy signs on FLAIR images before and after STA-MCA anastomosis in patients with Moyamoya disease. Acta Radiol. 2011; 52:291–296. PMID: 21498365.

7. Iida H, Itoh H, Nakazawa M, Hatazawa J, Nishimura H, Onishi Y, et al. Quantitative mapping of regional cerebral blood flow using iodine-123-IMP and SPECT. J Nucl Med. 1994; 35:2019–2030. PMID: 7989987.
8. Kawamura Y, Ashizaki M, Saida S, Sugimoto H. Usefulness of rate of increase in SPECT counts in one-daymethod of N-isopropyl-4-iodoamphetamine [123I] SPECT studies at rest and after acetazolamide challenge using amethod for estimating time-dependent distribution at rest. Ann Nucl Med. 2008; 22:457–463. PMID: 18600426.

9. Kawashima M, Noguchi T, Takase Y, Nakahara Y, Matsushima T. Decrease in leptomeningeal ivy sign on fluid-attenuated inversion recovery images after cerebral revascularization in patients with moyamoya disease. AJNR Am J Neuroradiol. 2010; 31:1713–1718. PMID: 20466798.

10. Kawashima M, Noguchi T, Takase Y, Ootsuka T, Kido N, Matsushima T. Unilateral hemispheric proliferation of ivy sign on FLAIR image in Moyamoya disease correlates highly with ipsilateral hemispheric decrease of cerebrovascular reserve. AJNR Am J Neuroradiol. 2009; 30:1709–1716. PMID: 19713323.

11. Komiyama M, Nakajima H, Nishikawa M, Yasui T, Kitano S, Sakamoto H. Leptomeningeal contrast enhancement in moyamoya : its potential role in postoperative assessment of circulation through the bypass. Neuroradiology. 2001; 43:17–23. PMID: 11214642.

12. Lee SK, Kim DI, Jeong EK, Kim SY, Kim SH, In YK, et al. Postoperative evaluation of moyamoya disease with perfusion-weighted MR imaging : initial experience. AJNR Am J Neuroradiol. 2003; 24:741–747. PMID: 12695215.
13. Maeda M, Tsuchida C. "Ivy sign" on fluid-attenuated inversion-recovery images in childhood moyamoya disease. AJNR Am J Neuroradiol. 1999; 20:1836–1838. PMID: 10588105.
14. Maeda M, Yamamoto T, Daimon S, Sakuma H, Takeda K. Arterial hyperintensity on fast fluid-attenuated inversion recovery images : a subtle finding for hyperacute stroke undetected by diffusion-weighted MR imaging. AJNR Am J Neuroradiol. 2001; 22:632–636. PMID: 11290469.
15. Mori N, Mugikura S, Higano S, Kaneta T, Fujimura M, Umetsu A, et al. The leptomeningeal "ivy sign" on fluid-attenuated inversion recovery MR imaging in Moyamoya disease : a sign of decreased cerebral vascular reserve? AJNR Am J Neuroradiol. 2009; 30:930–935. PMID: 19246527.

16. Mugikura S, Takahashi S, Higano S, Shirane R, Kurihara N, Furuta S, et al. The relationship between cerebral infarction and angiographic characteristics in childhood moyamoya disease. AJNR Am J Neuroradiol. 1999; 20:336–343. PMID: 10094366.
17. Narisawa A, Fujimura M, Tominaga T. Efficacy of the revascularization surgery for adult-onset moyamoya disease with the progression of cerebrovascular lesions. Clin Neurol Neurosurg. 2009; 111:123–126. PMID: 18995956.

18. Noguchi K, Ogawa T, Inugami A, Fujita H, Hatazawa J, Shimosegawa E, et al. MRI of acute cerebral infarction : a comparison of FLAIR and T2-weighted fast spin-echo imaging. Neuroradiology. 1997; 39:406–410. PMID: 9225318.

19. Ohta T, Tanaka H, Kuroiwa T. Diffuse leptomeningeal enhancement, "ivy sign," in magnetic resonance images of moyamoya disease in childhood. Case report. Neurosurgery. 1995; 37:1009–1012. PMID: 8559324.

20. Ringelstein EB, Sievers C, Ecker S, Schneider PA, Otis SM. Noninvasive assessment of CO2-induced cerebral vasomotor response in normal individuals and patients with internal carotid artery occlusions. Stroke. 1988; 19:963–969. PMID: 3135641.

21. Ringelstein EB, Van Eyck S, Mertens I. Evaluation of cerebral vasomotor reactivity by various vasodilating stimuli : comparison of CO2 to acetazolamide. J Cereb Blood Flow Metab. 1992; 12:162–168. PMID: 1727137.

22. Settakis G, Molnár C, Kerényi L, Kollár J, Legemate D, Csiba L, et al. Acetazolamide as a vasodilatory stimulus in cerebrovascular diseases and in conditions affecting the cerebral vasculature. Eur J Neurol. 2003; 10:609–620. PMID: 14641504.

23. Tomsick TA, Khatri P, Jovin T, Demaerschalk B, Malisch T, Demchuk A, et al. Equipoise among recanalization strategies. Neurology. 2010; 74:1069–1076. PMID: 20350981.

Fig. 1
The ivy sign is referred to as leptomeningeal high-signal intensity on FLAIR images along cerebral sulci. White arrows indicate ivy signs on bilateral hemispheres of moyamoya patient (A). Regions on bilateral hemispheres were classified into four territories16) (AJNR Am J Neuroradiol 20 : 336-343, 1999) : Red line - ACA, Green line - the anterior-MCA, Pupple line - the posterior MCA, Yellow line - PCA (B). ACA : Anterior cerebral artery, MCA : Middle cerebral artery, PCA : posterior cerebral artery, FLAIR : fluid-attenuated inversion recovery.
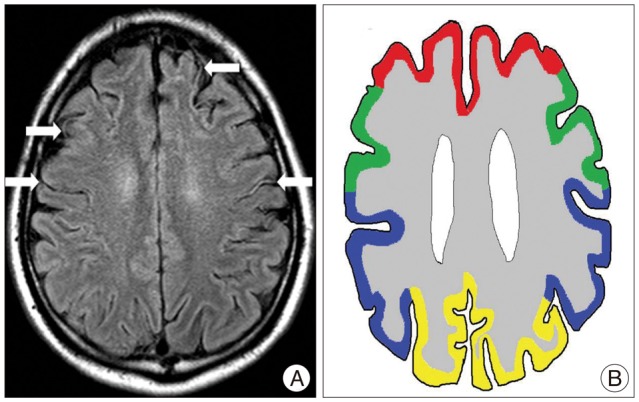
Fig. 2
A 36-year-old man with moyamoya disease who got the bilateral STA-MCA anastomosis surgery in our hospital. In the preoperative TFCA image, white arrows indicate moyamoya vessels on bilateral ICA (A). No vascular supply to intracranial region via ECA (B). STA-MCA anastomosis was done on bilateral hemisphere and well intracranial blood flow was checked from STA to MCA via anastomosis site in the postoperative TFCA image (C). TFCA : transfemoral cerebral angiography, STA : superficial temporal artery, ICA : internal carotid artery, ECA : external carotid artery, MCA : middle cerebral artery.
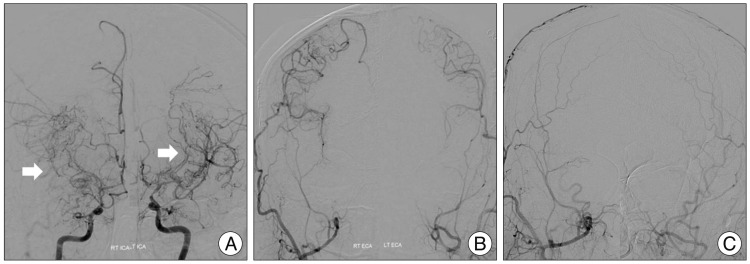
Fig. 3
Same patient with Fig. 2. Ivy sign was checked on bilateral hemispheres by indicating white arrows (A) and compared preoperative MR with postoperative MR FLAIR image, ivy sign was disappeared on bilateral hemispheres by indicating white arrows (B). Like MR FLAIR images, compared with preoperative SPECT and postoperative SPECT, CVR was increased on bilateral hemisphere before surgical treatment by indicating white arrowheads (C and D). SPECT : single photon emission computerized tomography, CVR : cerebrovascular reserve, FLAIR : fluid-attenuated inversion recovery.

Fig. 4
A 64-year-old woman with moyamoya disease. White arrowheads indicate the decreased CVR with SPECT images (A). The patient got the surgery, and decreased CVR was normalization on SPECT images after 1 month (B). CVR : cerebrovascular reserve, SPECT : single photon emission computerized tomography.
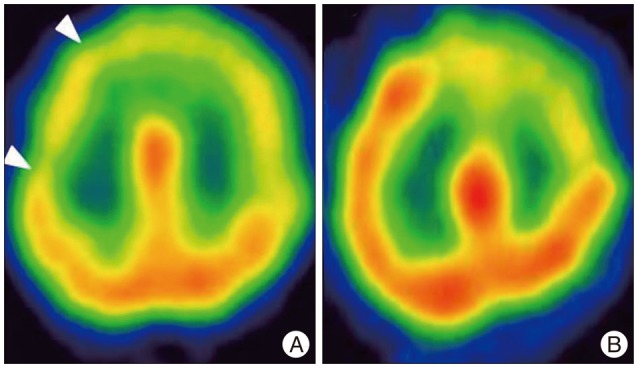
Fig. 5
Same patient with Fig. 4. We compared ivy sign on MR FLAIR image with MTT on MR perfusion image. Preoperatively, white arrows indicate the ivy signs on MR FLAIR image (A) and those were decreased on MR FLAIR images after surgery (B). MTT prolongation was observed on MR perfusion images in the right MCA territory in the preoperative state by indicating white arrows, and after surgery, MTT prolongation was improved on MR perfusion images like ivy signs on MR FLAIR images (C and D). MTT : mean transit time, FLAIR : fluid-attenuated inversion recovery.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download