Abstract
Spontaneous bilateral cerebellar infarction in the territory of the superior cerebellar arteries is extremely rare. Occasionally there have been reports of bilateral cerebellar infarction due to vertebrobasilar atherosclerotic occlusion or stenosis, whereas no report of bilateral cerebellar infarction due to complicated hemodynamic changes. In this report, we present a patient with bilateral cerebral infarctions related to stenoses of bilateral internal carotid arteries, in whom vertebrobasilar system was supplied by multiple collaterals from both posterior communicating arteries and right external carotid artery. We performed stent-angioplasty of bilateral internal cerebral arterial stenosis, and then acute infarction developed on bilateral superior cerebellar artery territories. The authors assumed that the infarction occurred due to hemodynamic change between internal carotid artery and external carotid artery after stent-angioplasty for stenosis of right internal carotid artery.
A number of collateral circulations exist between the internal and external cerebral circulation10,17). These become more apparent in pathological conditions such as vascular occlusive diseases6,8,16). For example, diminished flow in the basilar artery (BA) or vertebral artery (VA) without any symptoms of vertebrobasilar insufficiency suggested that posterior circulation was supplied from single or multiple collaterals from internal cerebral artery (ICA), external cerebral artery (ECA), and/or etc. Meanwhile, stent-angioplasty for carotid stenosis is well known about its effect on cerebral hemodynamics11,12). However, if ICA flow is increased abruptly as a result of stent-angioplasty, it may cause reduction of ECA flow relatively. These hemodynamic considerations are very important, when the vital areas are supplied by ECA branches.
We present a patient with bilateral ICA stenosis in whom most of the posterior circulation was supplied from posterior communicating artery (PComA) and the right ECA with arteriosclerotic steno-occlusion of both VAs. We experienced an unexpected complication of bilateral superior cerebellar artery (SCA) territorial infarction after stent-angioplasty for ICA stenosis, and this complication has never been reported before in our review of literatures.
A 75-year-old female patient visited our hospital with bilateral weakness (left>right) which had suddenly developed and persisted for 15 days. She also had hypertension and unstable angina which had been treated with antihypertensive and antiplatelet medications for 10 years. She had no history of diabetes mellitus, hyperlipidemia, and smoking, etc.
On neurological examination, bilateral hemiparesis (left : Grade IV, right : Grade IV+) was detected. The brain computed tomography scan showed a low-density area only in the right post-central gyrus, and diffusion weighted-magnetic resonance imaging (d-MRI) showed bilateral anterior cerebral artery-middle cerebral artery borderzone infarction (Fig. 1), and MR angiography showed high grade stenosis of both proximal ICA (Fig. 2).
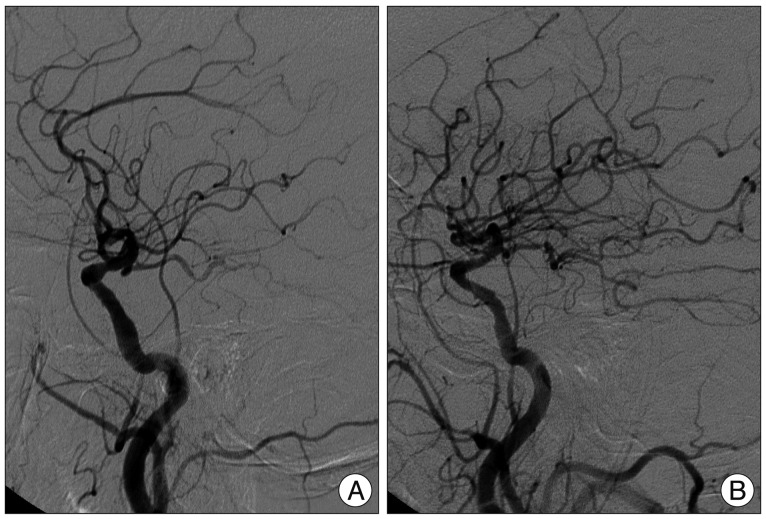
Catheter angiography was performed 4 days after admission. Common carotid angiography showed 85% stenosis of the left ICA origin (Fig. 3A) and 90% stenosis of right ICA origin (Fig. 3B). Vertebral angiography showed total occlusion of the right VA and multifocal stenosis of the left VA with faint flow to basilar artery (BA) (Fig. 4). PComAs on both sides were well visualized and filling to both posterior cerebral arteries (PCAs) (Fig. 5). However, retrograde flow to BA was not seen. Meanwhile, right occipital artery (OA) from right ECA supplied right VA and then BA to basilar tip (Fig. 3B).
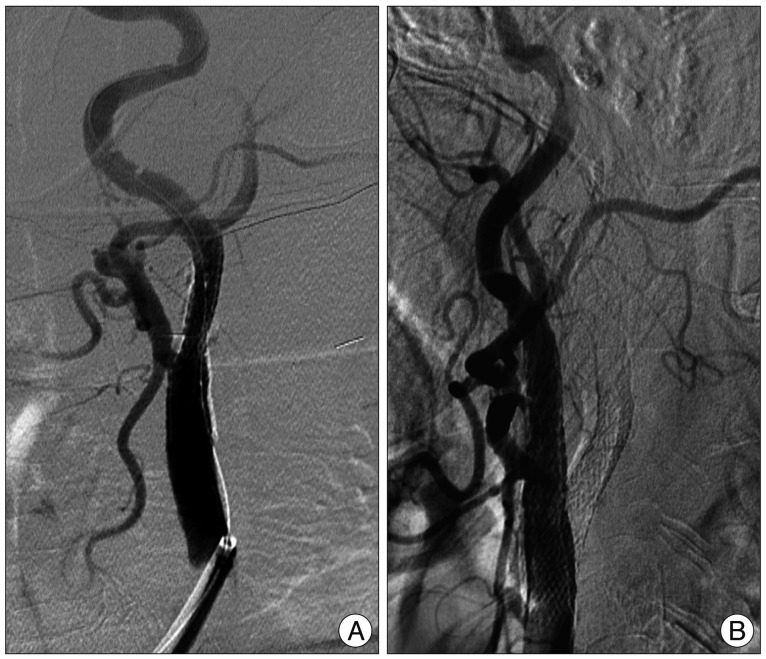
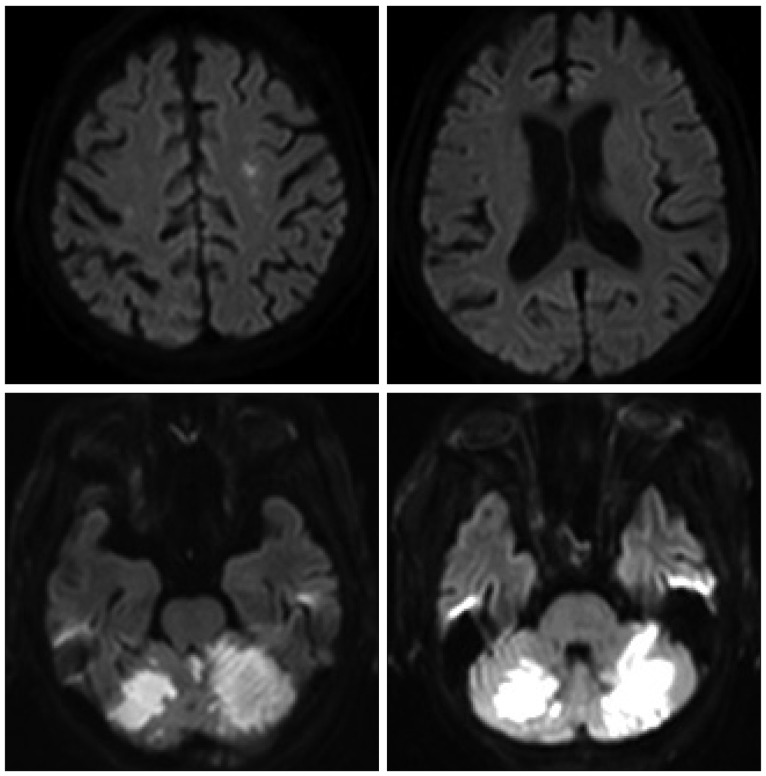
We performed stent-angioplasty for left ICA stenosis first under local anesthesia immediate after the catheter angiography (Fig. 6A). During the procedure no remarkable events took place, and the neurologic symptoms of the patient remained unchanged after the procedure. Two days after stent-angioplasty for left ICA stenosis, treatment for right ICA lesion was performed under local anesthesia (Fig. 6B). During the procedure no remarkable events took place, and the neurologic symptoms of the patient remained unchanged immediate after the procedure. After the 2nd session of angioplasty, we control the patient's systolic blood pressure (BP) about 120 mm Hg because of the hyperperfusion syndrome prevention. However, the consciousness level of the patient was getting worse after the procedure from alert to stuporous state. Two days after the 2nd procedure, d-MRI was performed and it showed acute cerebellar infarction in the territory of both SCAs, but no other newly developed infarction on both cerebral hemispheres (Fig. 7).
In general, spontaneous bilateral cerebellar infarction is very rare5,14,15). Even when it occurs, the majority of causes are vertebrobasilar atherosclerosis. Meanwhile, as a complication of angioplasty for ICA stenosis, hemodynamic change usually causes the cerebral hyperperfusion syndrome1,3,7,9,13). However, in our case, bilateral SCA infarction was occurred as a complication after angioplasty for stenosis of the ICA. Therefore, we try to point on the causes of this complication.
The first possible reason of the infarction is thromboembolism during the procedure of stent angioplasty2,4). However, the thromboembolism related to the procedure occurred in the same arterial territory in general. And, the cerebellar infarction due to thromboembolism usually occurs at the territories of posterior inferior cerebellar arteries (PICA) rather than anterior inferior cerebellar arteries or SCA, because PICA is more proximal and relatively larger size than the others. Besides, thromboembolism usually presents as multiple scattered small infarctions on d-MRI rather than a single large territorial infarction. However, in our case, infarction occurred at the territories of both SCA territories and presented as large infarctions limited to the territories of SCA, not in the territories of ICA or PICA. Therefore, we suggest that the thromboembolism can be ruled out as the cause of this cerebellar infarction.
The second and more likely cause is complicated hemodynamic changes. In this patient, vertebral angiography showed total occlusion of the right VA and faint flow of the left VA due to multifocal severe stenosis, whereas the OA from right ECA make up main collaterals for insufficient vertebrobasilar system as far as basilar tip, because both PCAs were supplied by both PComAs. Besides, there was no retrograde flow from PComA and PCA to BA before and after angioplasty for both ICA stenoses. If some anatomical continuity existed between PCA and BA, retrograde flow from PComA and PCA to BA might be seen after angioplasty for both ICA. However, the retrograde flow was not seen after the increase of both ICA flow. Therefore, both SCAs may be a kind of end artery and both SCA territories may be a kind of border zone in the vertebrobasilar system in this peculiar circumstance. Such being the case, abrupt increase of the right ICA flow by stent angioplasty might result in sudden decrease of right ECA flow and subsequently vertebrobasilar flow i.e., a kind of steal phenomenon. Of course, flow compromise of the right ECA might also be caused by jail effect of the stent and atheroma remodeling of the right proximal ICA to ECA orifice. However, these considerations did not appear to be proved clearly by the angiograms after angioplasty. Another necessary consideration is post-angioplasty BP control. Until the 2nd session, we did not control patient's BP without hypertension medications, and the mean systolic BP was about 140 mm Hg. However, after both ICA angioplasties, the patient's systolic BP was maintained about 120 mm Hg for protection of the hyperperfusion syndrome. This lowering BP was a possible aggravating factor that contributed to the patient's vertebrobasilar ischemic condition. We suggested that the SCA infarction was developed as a consequence of these sudden complicated hemodynamic changes like as a borderzone infarction.
The stent angioplasty may result in an abrupt and artificial hemodynamic change, thus careful evaluation and consideration of each patient's hemodynamic circumstances is very important before the treatment decision, including collateral circulations even remote circulations from the lesion.
References
1. Grunwald IQ, Politi M, Reith W, Krick C, Karp K, Zimmer A, et al. Hyperperfusion syndrome after carotid stent angioplasty. Neuroradiology. 2009; 51:169–174. PMID: 19104793.

2. Jang HJ, Cho KY, Lim JS, Park SK, Lee RS. Cerebral infarction after transfemoral carotid angiography : report of 2 cases. Korean J Cerebrovasc Surg. 2008; 10:528–531.
3. Kim DE, Choi SM, Yoon W, Kim BC. Hyperperfusion syndrome after carotid stent-supported angioplasty in patients with autonomic dysfunction. J Korean Neurosurg Soc. 2012; 52:476–479. PMID: 23323169.

4. Kim DS, Kim JK, Yoo DS, Huh PW, Cho KS, Kang JK. Complications of cerebral revascularization surgery. Korean J Cerebrovasc Dis. 2001; 3:50–53.
5. Kiroğlu Y, Karabulut N, Oncel C, Akdogan I, Onur S. Bilateral symmetric junctional infarctions of the cerebellum : a case report. Surg Radiol Anat. 2010; 32:509–512. PMID: 19760056.

7. Meyers PM, Phatouros CC, Higashida RT. Hyperperfusion syndrome after intracranial angioplasty and stent placement. Stroke. 2006; 37:2210–2211. PMID: 16888253.

8. Müller M, Schimrigk K. Vasomotor reactivity and pattern of collateral blood flow in severe occlusive carotid artery disease. Stroke. 1996; 27:296–299. PMID: 8571426.

9. Rezende MT, Spelle L, Mounayer C, Piotin M, Abud DG, Moret J. Hyperperfusion syndrome after stenting for intracranial vertebral stenosis. Stroke. 2006; 37:e12–e14. PMID: 16339466.

10. Romero JR, Pikula A, Nguyen TN, Nien YL, Norbash A, Babikian VL. Cerebral collateral circulation in carotid artery disease. Curr Cardiol Rev. 2009; 5:279–288. PMID: 21037845.

11. Sánchez-Arjona MB, Sanz-Fernández G, Franco-Macías E, Gil-Peralta A. Cerebral hemodynamic changes after carotid angioplasty and stenting. AJNR Am J Neuroradiol. 2007; 28:640–644. PMID: 17416813.
12. Schechter MM. The occipital-vertebral anastomosis. J Neurosurg. 1964; 21:758–762. PMID: 14210007.

13. Shim JH, Rha HK, Kim SR, Joo WI, Kim MC, Choi CR. Hyperperfusion syndrome after extracranial-intracranial bypass surgery. J Korean Neurosurg Soc. 2003; 34:526–530.
14. Tada Y, Mizutani T, Nishimura T, Tamura M, Mori N. Acute bilateral cerebellar infarction in the territory of the medial branches of posterior inferior cerebellar arteries. Stroke. 1994; 25:686–688. PMID: 8128527.

15. Tsukamoto T, Seki H, Saitoh J, Watanabe K. A case of bilateral cerebellar peduncle infarction. Jpn J Med. 1991; 30:376–378. PMID: 1942654.

16. Vernieri F, Pasqualetti P, Matteis M, Passarelli F, Troisi E, Rossini PM, et al. Effect of collateral blood flow and cerebral vasomotor reactivity on the outcome of carotid artery occlusion. Stroke. 2001; 32:1552–1558. PMID: 11441200.

17. Yamamoto S, Watanabe M. Novel collateral connecting the external and internal carotid arteries. Clin Anat. 2004; 17:70–72. PMID: 14695593.

Fig. 1
Diffusion weighted-magnetic resonance imaging at admission, showing bilateral anterior cerebral artery-middle cerebral artery borderzone infarction.
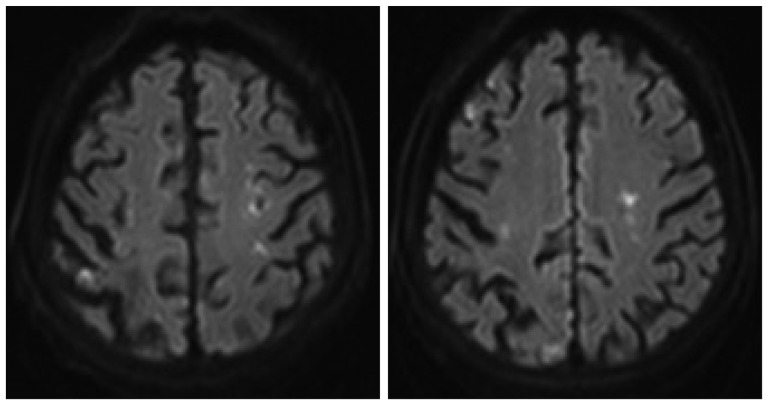
Fig. 2
Magnetic resonance angiogram, showing high grade stenoses of both proximal internal cerebral arteries.
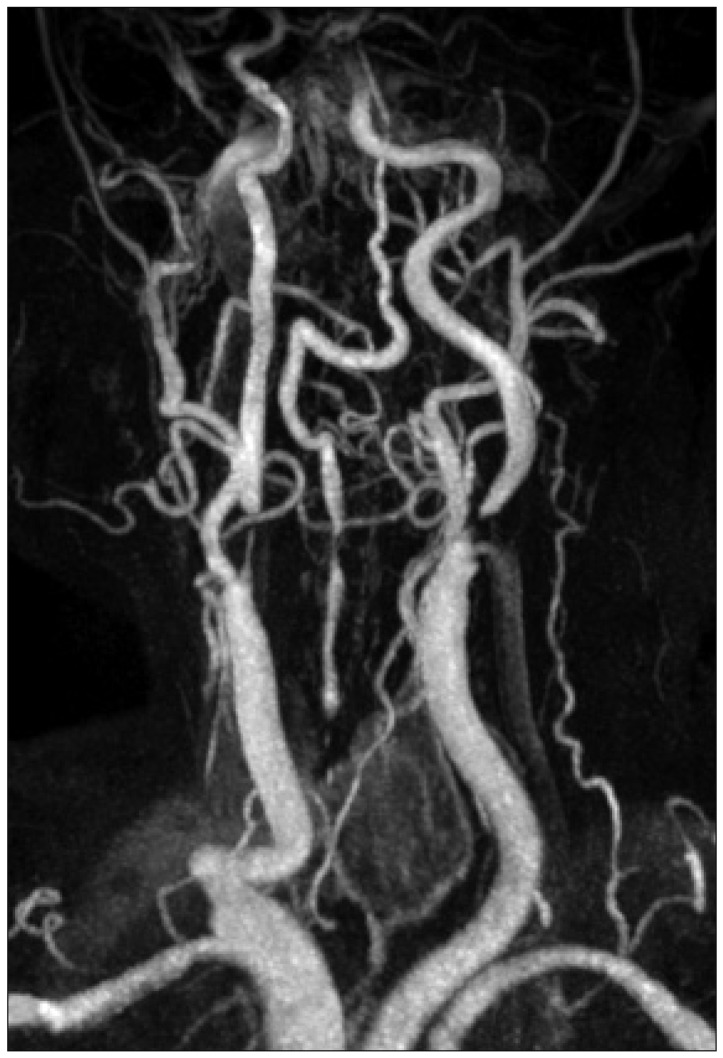
Fig. 3
Left common carotid angiogram (A), showing 85% stenosis of the left proximal internal cerebral artery (ICA). Right common carotid angiogram (B), showing 90% stenosis of the right proximal ICA, and right occipital artery from right external cerebral artery supplying right vertebral artery and then basilar artery to basilar tip.
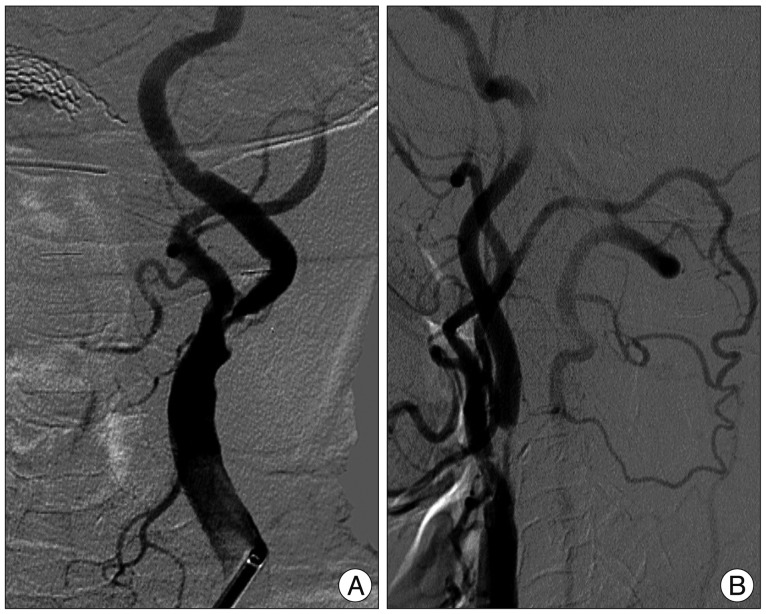
Fig. 4
Left subclavian angiogram, showing multifocal stenosis of the left vertebral artery with faint flow to basilar artery.
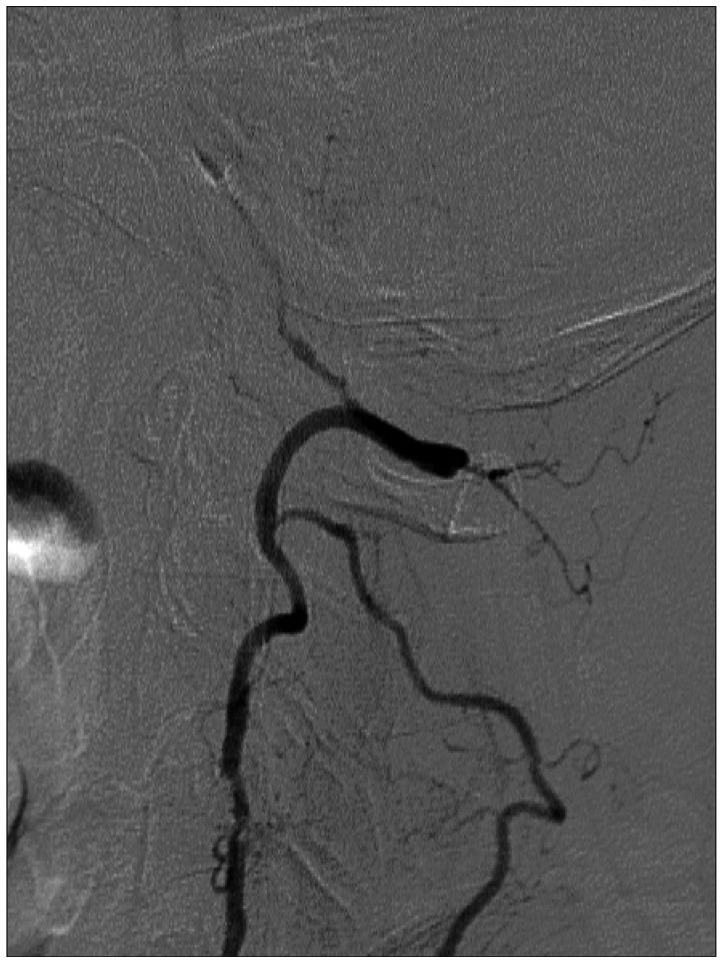
Fig. 5
Left internal cerebral artery angiograms (ICA) (A) and right ICA angiogram (B), showing well visualized posterior communicating arteries to posterior cerebral arteries, but no retrograde filling to basilar artery.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download