MATERIALS AND METHODS
Head X-ray computed tomography scans
Our practice has been to obtain serial head CT scans in all patients with traumatic ASDH. Head CT scans are performed at initial arrival, 2-4 hours after arrival, within 7 days after admission, and when symptoms worsen. This protocol has allowed us to follow the progression of traumatic ASDH.
CT sections (consecutive 4.5 mm slices) were obtained in an axial plane parallel to the orbitomeatal line.
Patient population
The database at our institute included all head traumatic patients admitted over a 7-year period (from 1 January 2005 to 31 July 2011). Initially, 742 ASDH patients were identified, but 113 were excluded because the main lesion was not ASDH. Of the remaining 629 patients, 329 patients underwent emergency surgery after an initial head CT scan, and 31 patients underwent emergency surgery after a second head CT scan (within 4 hours after the initial head CT scan) due to hematoma aggravation or brain swelling. In 41 patients who required emergency surgery, guardians refused surgery or surgery was not possible due to poor general condition. Based on initial or second head CT scans taken within 4 hours after arrival, our guidelines for emergency surgery were; 1) ASDH >10 mm thick or a midline shifting >5 mm with definite neurologic deficit such as an inability of obey commands, an abnormal light reflex, or motor weakness, 2) a comatose status [Glasgow Coma Scale (GCS) <9] with an ASDH <10 mm thick and a midline shifting <5 mm, and 3) aggravation of hematoma or a neurologic symptom within 4 hours of arrival, for example, a GCS decrease of >2 with an increase in hematoma or brain swelling by second head CT.
After excluding 401 patients that required initial surgery, 177 patients of the remaining 228 patients met the inclusion criteria and constituted the study cohort. The inclusion criteria adopted were; 1) initially non-operated ASDH, 2) initially mild neurologic symptoms (GCS ≥10), 3) supratentorial traumatic ASDH, and 4) a time of <4 hours between trauma and arrival. The exclusion criteria were; 1) no definite association between hematoma/edema and neurologic symptoms, such as, diffuse axonal injury, 2) spontaneous ASDH, 3) bilateral ASDH, 4) a history of a cranial operation, 5) an age of <15 years, 6) aggravated or expired patients due to co-morbid condition or complication, and 7) discharge or transfer before recovery.
The patients were divided into four groups according to clinical course. Of the 177 patients, 136 (76.8%) were allocated to the spontaneous resolution group (SRG; spontaneous resolution mainly between 1 week and 4 weeks after onset), 12 (6.8%) to the rapid worsening group (RWG; rapid worsening to the extent of requiring decompression surgery between 4 hours and 7 days after onset), 24 (13.6%) to the subacute worsening group (SWG; an increase in subdural fluid collection or hematoma sufficient to require surgery between 1 week and 4 weeks after onset), and 5 (2.8%) to the delayed worsening group (DWG; delayed aggravation such as hydrocephalus or subdural hygroma at >1 month after onset sufficient to require surgery).
Data collection
Paramedic field notes, emergency department records, admission notes, progress notes, outpatient notes, and serial head CT scans were reviewed in all patients.
The following data were collected; demographic data including sex and age, mechanism of injury including fall from standing, fall from a height, assault, in-car traffic accident, pedestrian traffic accident, motorcycle accident, or bicycle accident, co-morbid conditions including hypertension, diabetes mellitus, renal disease, ischemic heart disease, liver disease, stroke, cancer, epilepsy, or alcohol abuse, medication history of aspirin, antiplatelet drug, or anticoagulation therapy (e.g., warfarin), GCS scores on admission and at last follow-up, and associated intracranial injuries including cerebral contusion, subarachnoid hemorrhage (SAH), skull fracture, pneumocephalus, epidural hemorrhage (EDH), intraparenchymal hemorrhage (IPH), or intraventricular hemorrhage (IVH). Cerebral contusion was defined as petechial-appearing and ill-defined areas of mixed attenuation on CT scans, and IPH was defined as solid-appearing, discrete, and well-circumscribed hematoma
9). SAHs included both cisternal and sulcal SAHs.
The following findings were checked based on initial head CT; side of hematoma (e.g., right or left), dominant location of hematoma (e.g., frontal, temporal, parietal, or occipital), maximum thickness of hematoma, and midline shifting. Midline shifting was defined as distance between the most displaced midline structure and the midline of the skull.
Initial hematoma density was described as homogeneous or mixed. Homogeneous ASDH was defined when Hounsfield units (HUs) were in the range 50 to 100, and mixed ASDH was defined when HUs in the ranges 1 to 40 and 50 to 100 were mixed
2). Initial longitudinal proportion of largest hematoma, which was defined as the ratio of hematoma convexity to cerebral hemisphere convexity, was calculated (
Fig. 1).
 | Fig. 1Longitudinal proportion of the largest hematoma was defined as the ratio of hematoma convexity to cerebral hemisphere convexity (A/B). 
|
Initial number of CT slices containing hematoma was evaluated. If the longitudinal hematoma size for a particular slice was greater than 75% of the largest hematoma, the slice was considered 1 hematoma slice, if the hematoma size of a slice was approximately 25% to 75% of the largest hematoma, the slice was considered half a hematoma slice, and if the hematoma size of a slice was less than 25% of the largest hematoma, it was not considered a hematoma slice.
In addition, estimated hematoma volume index was evaluated. It was calculated as maximum thickness (mm)×longitudinal proportion×number of CT slices containing hematoma.
Finally, brain atrophy was evaluated. Quantitative atrophy including frontal horn index (FHI) and sylvian fissure ratio (SFR), and qualitative cortical atrophy which was classified as normal, mild, moderate, or severe were measured (
Fig. 2)
2).
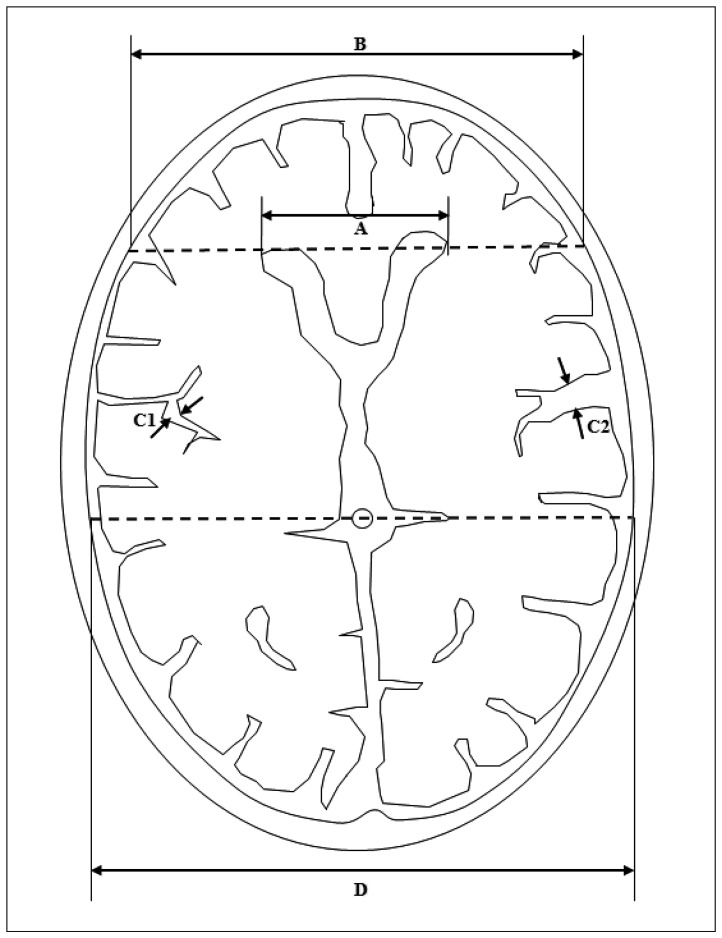 | Fig. 2Measurement of frontal horn index (FHI), and sylvian fissure ratio (SFR). A : Frontal horn diameter (FHD), the distance between the tips of the frontal horns measured on the CT section depicting them best. B : Innner table diameter (ITD), the distance between the inner tables of the skull measured on the same section, and along the same line used to measure FHD. C1 and C2 : Maximum widths of the two sylvian fissures. D : Transpineal coronal inner table diameter. FHI was defined as the ratio of FHD to ITD (A/B). SFR was defined as the average of the maximum width of the two sylvian fissures on the section showing them at their widest, divided by the transpineal coronal inner table diameter [(C1+C2)/2D]. If the sylvian fissure was not visible in the hematoma side, the contralateral side×2 was used. 
|
Statistical analysis
SPSS version 14.0 (SPSS Inc., Chicago, IL, USA) was used to analyze all data. Pearson's chi square test, the independent two samples t-test, the paired samples t-test, one-way analysis of variance (ANOVA), and the bivariate correlation test were used depending on the characteristics of the variables being compared. Multivariate analysis was subsequently performed to identify independent prognostic factors. Statistical significance was accepted for p values of < 0.05.
Go to :

RESULTS
Patient demographics are listed in
Table 1. Mean age of the 177 study subjects was 55.5±16.7 years (range, 17-94), mean follow-up period was 3.8±2.0 years, and 66.7% were men. Mechanisms of injury were; 91 (45.8%) falls from standing, 25 (14.1%) pedestrian traffic accidents, 24 (13.6%) falls from a height, 18 (10.2%) motorcycle accidents, 8 (4.5%) assaults, 6 (3.4%) in-car traffic accidents, and 5 (2.8%) bicycle accidents. Mean initial GCS score was 13.2±1.1 and final GCS score was 14.1±2.7 (
p=0.002, paired samples t-test).
Table 1
Demographic data and mechanisms of injury in the four study groups
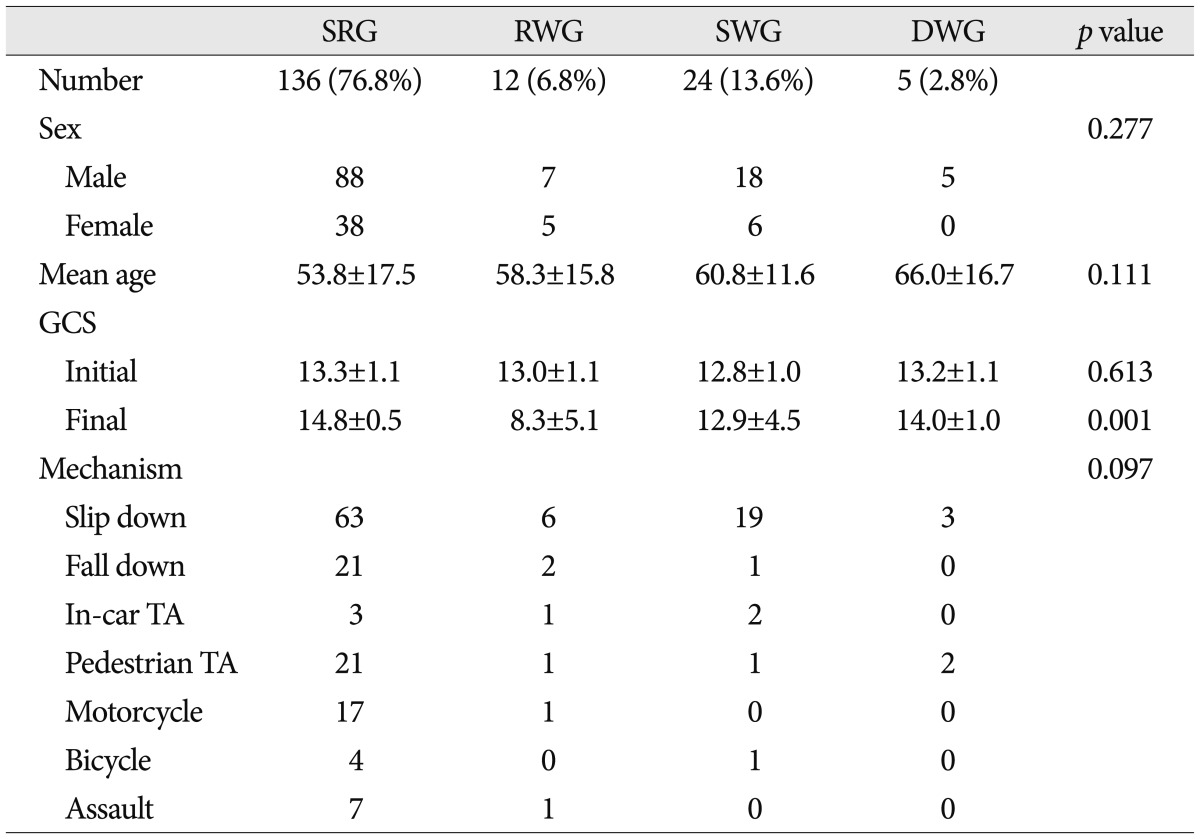

No significant intergroup difference was found for age, sex ratio, or mechanism of injury. Although mean age tended to be greater in the SWG and DWG than in the SRG and RWG, this was not significant (p=0.111, ANOVA and multivariate analysis).
Initial GCS scores were not significantly different between the four groups, but final GCS score was poorer in the RWG. In ascending order, final GCS scores increased significantly from RWG, SWG, DWG, to SRG (
p=0.001, ANOVA and multivariate analysis) (
Table 1). No correlation was found between initial GCS and final GCS scores (correlation coefficient 0.230, bivariate correlation test).
With regard to co-morbid conditions and medication history, only anticoagulation therapy was found to be significantly associated with clinical course (p=0.001, Pearson's chi square test).
Many patients exhibited additional intracranial injuries. Cerebral contusion and SAH were the most common, and were present in 89 (50.3%) and 71 (40.1%) patients respectively. Forty-seven (26.6%) patients had a skull fracture, 7 (4.0%) patients had pneumocephalus, 4 (2.3%) patients had IPH or IVH, and 1 (0.3%) patient had EDH. Cerebral contusion and SAH were significantly associated with clinical course, and interestingly, cerebral contusion (75.0%) and SAH (75.0%) were common in the RWG, whereas cerebral contusion (25.0%) and SAH (16.7%) were rare in the SWG (
p=0.024 for cerebral contusion and
p=0.006 for SAH, Pearson's chi square test) (
Table 2). In addition, cerebral contusion and SAH were found to be significantly related (
p=0.001, Pearson's chi square test), and the odds ratio was 20.2. Furthermore, cerebral contusion and SAH were correlated to severity of injury as estimated by initial GCS (
p=0.043 and 0.008 respectively, independent two samples t-test).
Table 2
Associated intracranial injuries and hematoma locations in the four study groups
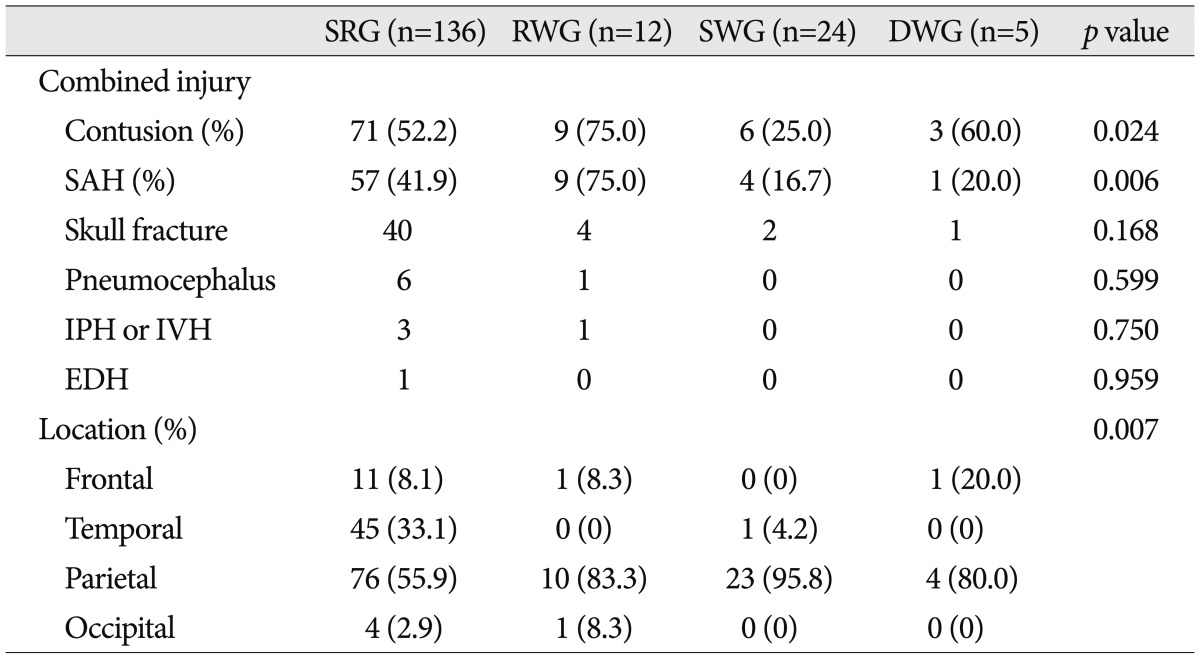

Side (right or left) of hematoma was not significantly different between the four groups.
Regarding the dominant location of hematoma, frequencies of occurrence followed the order; parietal (63.8%), temporal (26.0%), frontal (7.3%), occipital (2.8%). Hematoma location patterns were significantly different between the four groups. The parietal area dominated in the RWG (83.3%) and the SWG (95.8%), but accounted for a significantly smaller proportion in the SRG (55.9%) (
p=0.007, Pearson's chi square test) (
Table 2).
From the viewpoint of hematoma density, mixed density was more common in the RWG (66.7%) and the SWG (58.3%) than in the SRG (34.6%), in which homogeneous density was more common (65.4%) (
p=0.035, Pearson's chi square test) (
Table 3). On the other hand, mean age of mixed density patients (59.1±14.4) was significantly greater than that of homogeneous density patients (53.0±17.7) (
p=0.015, independent two samples t-test). Furthermore, mixed density was found to be significantly related to SFR and qualitative brain atrophy (
p=0.003, independent two samples t-test, and
p=0.001, Pearson's chi square test, respectively).
Table 3
Hematoma density and hematoma morphology in the four study groups
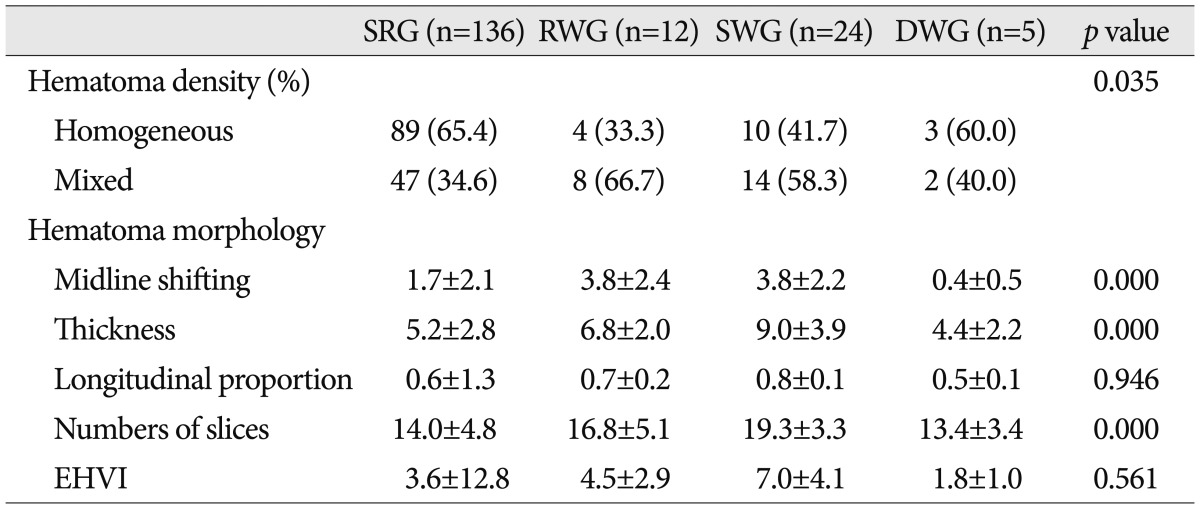

Midline shifting, hematoma thickness, and number of CT slices containing hematoma in the RWG and the SWG were significantly greater than in the SRG (
p=0.000, ANOVA and multivariate analysis), and midline shifting and hematoma thickness were significantly correlated (correlation coefficient 0.643, bivariate correlation test) (
Table 3).
However, longitudinal proportion of hematoma and estimated hematoma volume index were not significantly different between the four groups (
Table 3).
Concerning the brain atrophy, SFR and qualitative atrophy were significantly associated with clinical course, and were more severe in the SWG and the DWG than in the SRG and the RWG (
p=0.047 for SFR, by ANOVA and multivariate analysis, and
p=0.016 for qualitative atrophy, by Pearson's chi square test respectively) (
Table 4). However, FHI was not different in the four groups. SFR and qualitative atrophy well found to be strongly related, but no relation was found between FHI and qualitative atrophy (
p=0.002 and 0.548 respectively, ANOVA and multivariate analysis).
Table 4
Brain atrophy in the four study groups

In the SRG, almost all cases resolved during the subacute phase (i.e., between 1 week and 4 weeks after onset), and unusually, 2 cases resolved rapidly within 24 hours after onset.
In the RWG, craniotomy or craniectomy was required in all 12 patients. The mean time taken for progression to reach a situation requiring surgery was 2.1±1.2 days (range, 1-4). Two of the 12 patients died despite immediate the operations, and one died after refusal to undergo surgery.
In the SWG, hematomas were observed to undergo transient natural shrinkage during the acute phase (<1 week), but to progress to subacute or chronic SDH with severe midline shifting during the subacute phase. Three of the 24 patients underwent craniotomy, and 21 patients underwent trephination surgery. Mean time for progression to expansive subacute or chronic SDH requiring surgery was 12.7±3.3 days (range, 8-19). One patient died despite surgery, and 1 patient died due to refusal to undergo surgery.
In the DWG, 2 of the 5 patients developed hydrocephalus requiring a shunt operation, 2 developed subdural hygroma requiring trephination surgery, and 1 developed chronic SDH on the contralateral side. Mean time to progression to a delayed complication in this group was 5.6 months (range, 2-10).
The following case descriptions provide representative examples.
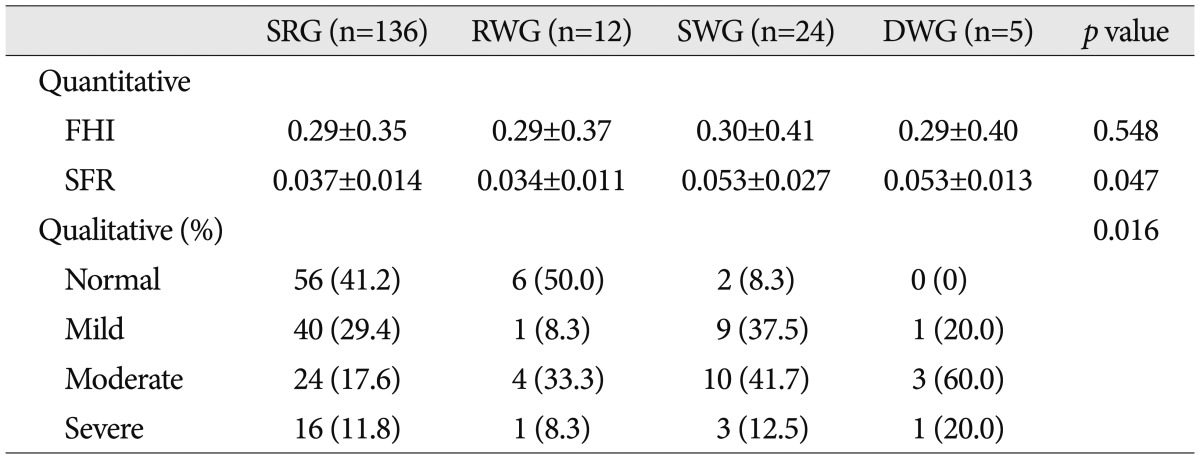
A 67-year-old woman presented with a GCS 13 after an in-car traffic accident. Initial head CT revealed a thick ASDH of mixed density, combined cerebral contusion and SAH, and moderate midline shifting. Three days later, with her neurologic deterioration, follow-up head CT revealed aggravated hematoma and midline shifting. Craniectomy was performed and her final GCS score was 10 (
Fig. 3).
 | Fig. 3Computed tomography (CT) scans showing an example of rapid worsening. A : Initial head CT reveals a mixed density, thick ASDH with cerebral contusion, traumatic SAH, and moderate midline shifting. B : Three hours later, head CT shows slight hematoma thinning and a midline shifting improvement. C : However, 3 days later, the patient showed a sudden neurologic deterioration (GCS score 8), and follow-up head CT reveals aggravated hematoma and midline shifting. Craniectomy was performed immediately. Her final GCS score was 10. ASDH : acute subdural hematoma, SAH : subarachnoid hemorrhage, GCS : Glasgow Coma Scale. 
|
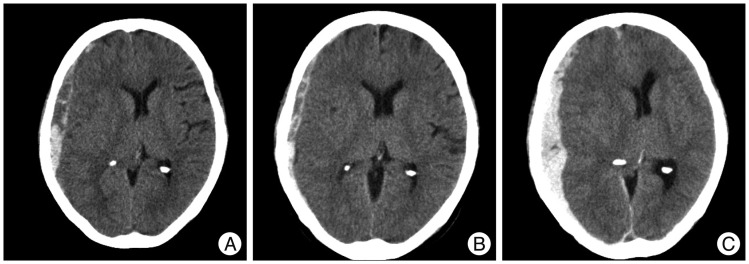
A 74-year-old man presented with a GCS score of 13 after a slip down. Initial head CT revealed a thick ASDH of mixed density with mild midline shifting. One week later, follow-up head CT showed hematoma resolution transiently. However, at 2-weeks after admission, the symptoms worsened gradually to a GCS score of 10, and head CT revealed low density subdural fluid collection with severe midline shifting. Trephination surgery was performed and her final GCS score was 14 (
Fig. 4).
 | Fig. 4Computed tomography (CT) scans showing an example of subacute worsening. A : Initial head CT reveals a mixed density, thick ASDH with mild midline shifting with moderate brain atrophy. B : One week later, head CT shows hematoma resolution transiently. C : However, at 2 weeks after admission, his symptoms worsened gradually to GCS score 10. Follow-up head CT reveals low-density subdural fluid collection with severe midline shifting. Trephination surgery was performed, and fortunately, his final GCS score recovered to 14. ASDH : acute subdural hematoma, GCS : Glasgow Coma Scale. 
|
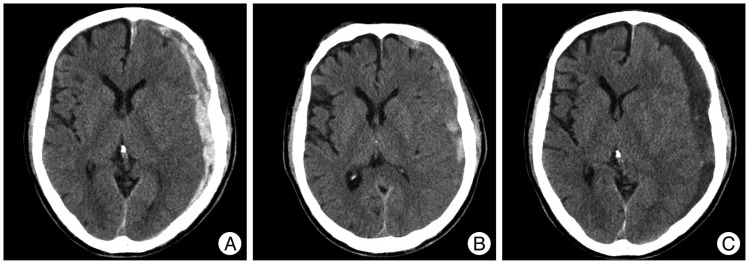
A 70-year-old man presented with a GCS score of 13 after a pedestrian traffic accident. Initial head CT revealed a thick ASDH of homogeneous density. Three months later, follow-up head CT revealed hydrocephalus, and then, ventriculo-peritoneal shunt was performed (
Fig. 5).
 | Fig. 5Computed tomography (CT) scans showing an example of delayed worsening. A : Initial head CT reveals a homogeneous density ASDH in the right frontal and interhemispheric fissure. B : Two weeks later, head CT shows hematoma resolution but progression of bilateral subdural hygroma. C : Three months later, follow-up head CT reveals hydrocephalus and a ventriculo-peritoneal shunt procedure was performed. ASDH : acute subdural hematoma. 
|
Go to :

DISCUSSION
Management decisions in cases of ASDH should take into account CT findings, neurologic symptoms, and general condition. When initial surgery is not needed, the outcomes of conservative treatment are satisfactory
7,
8), but worsening of initially non-operated ASDH is not uncommon
7,
8,
12,
19). The motive for this study arose from a recurring clinical question. Given a patient with initially non-operated traumatic ASDH, how can one predict whether the lesion will aggravate or not and if it does, what is the prognosis? This retrospective cohort study characterizes the natural course of non-operated traumatic ASDH and provides some risk factors for lesion aggravation to the extent of requiring surgery.
Demographics
In the present study, 23.2% of initially non-operated ASDH cases eventually required surgery. In particular, sudden worsening in the subacute phase (that is the SWG) was 13.6%. As mentioned above, in this group, the hematomas shrank transiently, and then, progressed to thick low density subdural fluid collections with marked mass effects. Consideration of this phenomenon is crucial to avoid complacency due to transient ASDH resolution during the acute phase.
As has been previously reported, sex ratio and mechanism of injury were not found to be correlated with clinical course
13,
22,
25). Some have reported a relation between worsening of initially non-operated ASDH and old age
12,
23). Somewhat surprisingly, although members of the SWG and the DWG tended to be older than members of other groups, no statistical significance was found in the present study, which contradicts previous reports.
Our finding of no difference of initial GCS between the four groups suggests that initial neurologic states and damage intensities were similar in the four study groups. However, based on final GCS scores and mortality ratios, outcomes were significantly poorer in the RWG and the SWG. Interestingly, these findings suggest that initial GCS scores were not predictive of ASDH progression.
In addition, neither the presence of a co-morbid condition including alcohol abuse, nor the use of aspirin and/or an antiplatelet drug appeared to influence the clinical course of ASDH. On the other hand, oral anticoagulants including warfarin worsened clinical course, as has been previously reported
13,
22,
25).
Associated intracranial injuries
In terms of associated intracranial injuries, two prognostic factors were found to be strongly associated with clinical course, namely, cerebral contusion and SAH. Curiously, cerebral contusion and SAH were common in the RWG, but rare in the SWG. The presence of cerebral contusion or traumatic SAH is perhaps a strong predictor of rapid worsening, which echoes the findings of some other authors
5,
6,
26).
Classically, there are two common types of traumatic ASDH resulted from two distinct pathologies. The first type of ASDH manifests as accumulation into the adjacent subdural space around a parenchymal brain contusion or laceration, and is usually associated with a severe underlying primary brain injury such as cerebral contusion or SAH.
Cerebral contusions are focal lesions of subpial hemorrhage and swelling, and sometimes are referred to as lacerations when overlying pia mater is compromised. Generally, contusions are caused by direct or indirect impact, and common in regions that contact bony surfaces in the cranial vault, such as, the frontal base or temporal pole. Occasionally, contusions can cause significant mass effects due to progressive surrounding edema or progression to an IPH during the acute phase (within 1 week of injury). Contusions also represent a significant source of secondary injury to adjacent tissue via the release of neurotransmitters and local biochemical changes
32).
Traumatic SAH has cisternal (basal cisterns, interhemispheric spaces, and Sylvian fissures) or sulcal (cortical convexity) distributions, and results from relatively severe injury to the brain; high angular acceleration of considerable duration is necessary to produce sufficient strain to rupture of superficial vessels in subarachnoid spaces. Post-traumatic vasospasm can also occur early (within 5 days), and induce focal or diffuse cerebral edema
32).
Accordingly, the presence of combined contusion or SAH with ASDH indicates the first type of ASDH associated with substantial impact and more severe brain parenchymal injury, and can worsen the course of the ASDH during the acute phase (within 1 week after onset) by the above-mentioned mechanisms. In fact, in the present study, presences of cerebral contusion or SAH were found to be significantly related, and correlated with severity of initial injury as estimated by initial GCS scores.
Unlike the first type of ASDH, the second type of ASDH is caused by inertial forces (acceleration or deceleration) that tear surface bridging vessels entering dural sinuses, and accumulates diffusely and thickly over the cerebral convexity. In general, the absence of combined contusion or SAH indicates this second type of ASDH associated with less severe brain parhenchymal injury. Primary brain damage may be less severe for this etiology, and a lucid interval may occur with subsequent subacute deterioration. In other words, because hematoma is diffuse and thick, and sometimes venous bleeding is continuous, hematoma resolution is relatively slow and may even be aggravated during the subacute phase (1 week and 4 weeks after onset)
32).
Hematoma location
Regarding hematoma locations in the four groups, a parietal location was found to be indicative of a poorer ASDH course. In particular, parietal area is prone to subdural bleeding because it contains more cortical vessels entering dural sinuses. In addition, because the subdural space extends over a more widespread field in the parietal area than in other brain areas, the compressive effect that suppresses hematoma accumulation is weakest. Accordingly, in the parietal area, it is easier to accumulate thick hematoma, and subsequently, more difficult to resolve spontaneously.
Hematoma density
From the viewpoint of hematoma density, mixed density was more common in the RWG and the SWG, whereas homogeneous density was more common in the SRG.
There are several suspected causes for mixed density. First, mixed hematoma density could be due to hyperacute hematoma produced by ongoing active bleeding or the onset of coagulopathy complicating the management of these lesions
10). Head CT scans demonstrate areas of high density corresponding to clotted hematoma, a mixed with areas of relatively low density, corresponding to liquid blood
10). This mixed density ASDH can exhibit deterioration in the acute phase (within 1 week after onset), due to rapid arterial hematoma accumulation, or in the subacute phase (1 to 4 weeks after onset), due to slow and steady venous hematoma accumulation
20).
Second, mixed hematoma density could also be due to cerebrospinal fluid (CSF) mixed hematoma. In this situation, the arachnoid membrane is frequently torn, which causes a mixture of CSF and blood to accumulate in the subdural space. Head CT scans reveal an expansive lesion with a low density area in the hematoma. This CSF mixture may exhibit rapid resolution within 24 hours due to dilution of the hematoma by CSF
1,
14,
15). On the other hand, steady CSF leakage from the subarachnoid space can cause worsening due to CSF accumulation during the subacute phase (1 to 4 weeks after onset).
The third cause of mixed hematoma density is acute-on-chronic SDH, which appears as a hyperdense layer of clot with expansive, irregular blurred margin or lumps in liquefied chronic hematoma
18,
23). Acute trauma on the patients with chronic SDH may develop acute subdural bleeding within chronic SDH. Generally, acute-on-chronic SDH tends to occur in elderly patients with brain atrophy
18,
30). In the present study, we speculate that the majority of mixed density ASDHs were acute-on-chronic SDHs, because mixed density ASDH was found to be strongly correlated with old age or brain atrophy. Since the potential subdural space is substantial and the bleeder is usually a venous capillary, symptoms can be mild despite thick hematoma
18). Furthermore, since chronic SDH is usually associated with excessive activation of the coagulation and fibrinolytic systems, acute bleeding into the hematoma cavity may not produce a solid clot and its spontaneous resolution may be difficult
24). As a result, acute-on-chronic SDH can cause subacute worsening (1 to 4 weeks after onset) due to disturbance of hematoma resolution.
Midline shifting & hematoma thickness
Not surprisingly, midline shifting, hematoma thickness, and number of CT slices containing hematoma were found to be predictors of worsening. Initial midline shifting may be resulted from thick hematoma or diffuse cerebral edema. Relationships between such factors and subsequent worsening suggest thin hematomas are in general stable, whereas thick hematomas are at risk of progression. We were somewhat surprised to find that longitudinal proportion of hematoma and estimated hematoma volume index were not predictive of progression. These results suggest that degrees of longitudinal spread and hematoma volume are not associated with clinical course.
Brain atrophy
Brain atrophy was severe in the SWG and the DWG, but mild in the SRG and the RWG. Some authors believe that the space created by premorbid cerebral atrophy contributes to the development of subacute fluid collection, delayed subdural hygroma, or delayed hydrocephalus
12,
23,
27). In other words, subacute fluid collection, delayed subdural hygroma, or delayed hydrocephalus could be presented as a compensatory increase in subdural space in intracranial hypotension caused by cortical atrophy
4,
11,
16,
17,
31).
On the other hand, the absence of brain atrophy could suppress the accumulation of hematoma or fluid collection as in the SRG, or paradoxically cause acute brain compression due to cerebral edema or hematoma because of the restricted free subdural space as in the RWG.
Summary of risk factors
Several conditions have been proposed for the spontaneous resolution of ASDH
15,
21,
29). In the present study, the factors for spontaneous resolution include no anticoagulant medication, homogeneous density, mild midline shifting (1.7±2.0 mm), thin hematoma (5.2±2.7 mm), fewer CT slices containing hematoma (14±4.7), and the absence of brain atrophy.
Rapid (within 1 week after onset) worsening of ASDH is often encountered clinically, and in the present study, the risk factors for rapid worsening were anticoagulation therapy, associated intracranial injury including cerebral contusion or SAH, a parietal location, mixed density, severe midline shifting (3.8±2.4 mm), thick hematoma (6.8±2.0 mm), many hematoma slices (16.8±5.1), and the absence of brain atrophy. In summary, we postulate three potential mechanisms for rapid worsening; 1) the rapid accumulation of ASDH due to cortical vessel rupture (i.e., hyperacute mixed density SDH), 2) the rapid aggravation of ASDH due to coagulopathy, 3) the rapid aggravation of brain swelling due to combined cerebral contusion or SAH.
Several previous studies have addressed subacute (1 to 4 weeks after onset) worsening of non-operated ASDH
12,
19,
23). In the present study, the factors found to predict subacute worsening were anticoagulation therapy, the absence of combined cerebral contusion or SAH, a parietal location, mixed density, severe midline shifting (3.8±2.2 mm), thick hematoma (9.0±3.8 mm), many hematoma slices (19.3±3.3), and brain atrophy. In summary, we postulate two possible mechanisms for subacute worsening; 1) delayed resolution of hematoma in conditions such as acute-on-chronic SDH or thick ASDH, 2) subacute CSF accumulation in the subdural space in conditions such as CSF mixed ASDH or brain atrophy.
Delayed (more than 1 month after onset) complications such as hydrocephalus and subdural hygroma were reported by some authors
4,
11,
16,
17,
31). In the present study, the only factors found to predict delayed worsening was brain atrophy.
Limitation
This study has several limitations that warrant consideration. Foremost, its retrospective nature is intrinsically prone to patient-selection bias. In addition, we only examined ASDH found in the acute traumatic period (within 4 hours after trauma), and therefore, our findings cannot be applied to ASDH arising many hours or even days after injury. In fact, a considerable portion of traumatic ASDHs develop after several hours and are often associated with coagulopathy
28).
Go to :









 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download