Abstract
Objective
To compare spinopelvic parameters in young adult patients with spondylolysis to those in age-matched patients without spondylolysis and investigate the clinical impact of sagittal spinopelvic parameters in patients with L5 spondylolysis.
Methods
From 2009 to 2012, a total of 198 young adult male patients with spondylolysis were identified. Eighty age-matched patients without spondylolysis were also selected. Standing lateral films that included both hip joints were obtained for each subject. Pelvic incidence (PI), sacral slope (SS), pelvic tilt, lumbar lordosis angle, sacral inclination, lumbosacral angle, and sacral table angle were measured in both groups. A comparative study of the spinopelvic parameters of these two groups was performed using SPSS 15.0 (SPSS Inc., Chicago, IL, USA).
Results
Among the aforementioned spinopelvic parameters, PI, SS and STA were significantly different between patients with spondylolysis and those without spondylolysis. PI and SS were higher in the spondylolysis group than in the control group, but STA was lower in the spondylolysis group than in the control group.
Conclusion
PI and SS were higher in the spondylolysis group than in the control group, but STA was lower in the spondylolysis group than in the control group. Patients with spondylolysis have low STA at birth, which remains constant during growth; a low STA translates into high SS. As a result, PI is also increased in accordance with SS. Therefore, we suggest that STA is an important etiologic factor in young adult patients with L5 spondylolysis.
Abnormal sagittal spinopelvic parameters may cause persistent back pain and be central to the development and progression of many spinal disorders, including spondylolysis, spondylolisthesis and a variety of other spinal pathologies. Recent studies have suggested an association between increased pelvic incidence (PI) and the development of spondylolytic spondylolisthesis. Increased pelvic incidence may also be related to the severity of spondylolisthesis3,6,10). Some correlation studies on spondylolisthesis have also found significant differences in the spinopelvic parameters of patients with spondylolisthesis and normal subjects2,7,9,13,15). However, these studies are somewhat limited because the two groups were not comparable in terms of age and the subjects were not recruited from a single institution.
The aims of this study were to compare spinopelvic parameters in young adult patients with spondylolysis to those in age-matched patients without spondylolysis. This study also investigated the clinical impact of sagittal spinopelvic parameters in patients with L5 spondylolysis.
From 2009 to 2012, a total of 198 young adult male patients with spondylolysis were identified. Spondylolysis was diagnosed by lumbar radiography and three-dimensional spine computed tomography. Eighty age-matched patients without spondylolysis were also selected. More detailed demographic data of both groups are shown in Table 1. To minimize confounding factors such as remodeling, secondary morphologic changes and age- and gender-related differences, only 18 to 24 year old young adult male patients were included. Exclusion criteria included dysplastic, degenerative spondylolisthesis, spondylolysis at L3 or L4, unilateral L5 spondylolysis, lumbosacral transitional vertebra, scoliosis, Scheuermann disease, hip pathology and indecipherable femoral heads on lateral lumbosacral radiographs. Patients with high-grade spondylolisthesis were also excluded because there can be significant dystrophic changes at the lumbosacral junction (sacral doming, L5 trapezoidal body), which makes it difficult to accurately measure angles involving the L5 or S1 endplates.
Standing lateral films that included both hip joints were obtained for each subject while the subject was placed in a standing position with their arms folded on their chest and knees fully extended. All radiographic measurements were calculated from the existing lateral lumbosacral radiographs and were performed by two spine surgeons using picture archiving and communication systems software. PI, sacral slope (SS) and pelvic tilt (PT) were measured as described by Legaye et al.8) Lumbar lordosis (LL) and sacral inclination were measured using the method described by Wiltse and Winter19) Lumbosacral kyphosis was evaluated by "lumbosacral" angle (LSA), which is the angle between the superior endplate of L5 and the posterior cortex of S1, as described by Dubousset1) To evaluate the tilting angle of the sacral endplate, the sacral table angle (STA) was measured between the line along the sacral endplate and the line drawn along the posterior aspect of the S1 vertebral body, according to the method of Osterman and Osterman11) A comparative study of the spinopelvic parameters of these two groups was performed using SPSS 15.0 (SPSS Inc., Chicago, IL, USA).
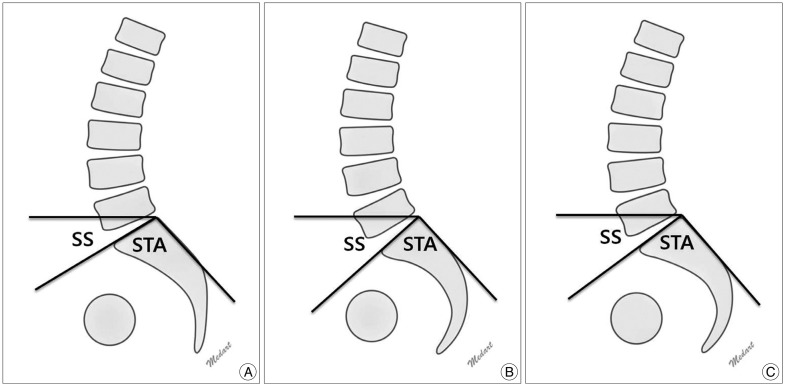
Among the aforementioned spinopelvic parameters, PI, SS and STA were significantly different between patients with spondylolysis and those without spondylolysis (Table 2). PI and SS were higher in the spondylolysis group than in the control group, but STA was lower in the spondylolysis group than in the control group (Fig. 1A, B). The mean PI values were 53.8±11.3 in the spondylolysis group and 45.8±7.2 in the control group (p<0.001). The mean SS values were 37.1±10.1 in the spondylolysis group and 29.3±7.0 in the control group (p<0.001). The mean STA values were 94.4±5.8 in the spondylolysis group and 100.5±4.7 in the control group (p<0.001).
Some authors have previously studied about the correlation of sagittal alignment between isthmic spondylolisthesis and normal control groups. Labelle et al.7) found that PI, SS and LL were much higher in subjects with spondylolisthesis than in healthy subjects while thoracic kyphosis (TK) was lower than average in subjects with spondylolisthesis. This was a retrospective multicenter study that involved 13 medical centers, and the authors did not measure STA or LSA. In 2006, Roussouly et al.13) also performed a retrospective review of 82 patients with spondylolysis. They compared these patients with a reference population of 160 normal adult volunteers without symptoms of back pain or radiographic abnormalities12). They demonstrated that patients with spondylolysis showed greater PI and LL, but less segmental extension of L5 on S1 than normal patients. Vialle et al.15,16) also compared sagittal alignment in a cohort of 244 patients with spondylolysis to sagittal alignment of a cohort of 300 healthy volunteers enrolled in a previously published study. They noted that PI, SS, PT and LL were significantly higher in patients with spondylolysis, but LSA and TK were significantly lower in patients with spondylolysis. The authors of these studies speculate that PI is an important factor in the etiology of spondylolysis.
In our study, PI and SS were higher in the spondylolysis group than in the control group. However, PT did not differ between the two groups. Because PI is the summation of SS and PT, PI is proportional to SS. Therefore, we suggest that the PI in patients with spondylolysis increased as SS increased. Our results were consistent with those of previous studies. Stagnara et al.14) reported that LL increases linearly with SS, and PI is the summation of SS and PT. PI, SS, and LL are correlated in patients with spondylolysis. LL increases as SS increases to maintain the center of gravity behind the femoral head and sustain balanced posture. Our results demonstrated that although PI and SS increased in the spondylolysis group, LL did not differ for the spondylolysis and control groups, unlike the results of previous studies. We postulate that the compensation mechanism for increasing LL have not yet been established because our patients are younger and show less slippage than the subjects of previous studies. Therefore, LL has not yet increased in our patients with spondylolysis. Labelle et al.5) reported that LL increases to keep the center of gravity and C7 plumb line behind the hips in patients with spondylolysis who showed high PI. This first compensation mechanism occurs by increasing intervertebral segmental lordosis and/or by including more vertebrae in the lordotic segment. Our study demonstrates that SS and PI increase in patients with spondylolysis. LL then also increases to maintain sagittal balance along the spondylolisthesis progression (Fig. 1C).
Another important result derived from our study is that STA is lower in the spondylolysis group than in the control group. In 2002, Inoue et al.4) reported that STA showed a statistically significant and progressive decrease when spondylolysis and subsequent spondylolisthesis were present. According to their study, STA had a close negative correlation with the degree of adolescent vertebral slippage in patients with L5 isthmic spondylolisthesis. In 2005, Whitesides et al.18) also concluded that patients with lower STA were more likely to develop a pars defect, and STA was more strongly associated with the occurrence of pars defects than PI. They suggested that upper sacral deformities appear due to a growth plate response to changed pressure gradients across the epiphyseal plate rather than due to torsional interosseous remodeling of the ilium and acetabular area. Thus, changes in PI are secondary to sacral deformity. Our results are concordant with the results of these studies. Additionally, Wang et al. reported that decreased STA was significantly related to the occurrence of L5-S1 spondylolisthesis. STA decreased with increased slippage severity of L5-S1 developmental spondylolisthesis in children and adolescents. They also found similarities in the sacral morphology (STA) of children and adolescents (skeletally immature) with those of the adult (skeletally mature) population. This suggested that sacral morphology measured by STA could be a more constant measure than pelvic morphology measured by PI. Based on our results, we also speculate that STA in patients with L5 spondylolysis is congenitally (or genetically) smaller, and STA is an important etiologic factor in young adult patients with L5 spondylolysis.
We suggest a possible mechanism that occurs during growth, in which patients with spondylolysis have low STA at birth. Low STA values are stable and little affected by bony adaptive changes in the process of growth. These values also translate into steeper SS with higher shear stress in order to maintain balance after patients stand erect. PI also increases secondarily to SS.
Through this comparative study, we concluded that PI and SS were higher in the spondylolysis group than the control group, but STA was lower in the spondylolysis group than the control group. Patients with spondylolysis have low STA at birth, which remains constant during growth; a low STA translates into high SS. As a result, PI is also increased in accordance with SS. Therefore, we suggest that STA is an important etiologic factor in young adult patients with L5 spondylolysis.
Acknowledgements
This paper was supported by Fund of Biomedical Research Institute, Chonbuk National University Hospital.
References
1. Dubousset J. Treatment of spondylolysis and spondylolisthesis in children and adolescents. Clin Orthop Relat Res. 1997; 77–85. PMID: 9137179.

2. Endo K, Suzuki H, Tanaka H, Kang Y, Yamamoto K. Sagittal spinal alignment in patients with lumbar disc herniation. Eur Spine J. 2010; 19:435–438. PMID: 20091188.

3. Hresko MT, Labelle H, Roussouly P, Berthonnaud E. Classification of high-grade spondylolistheses based on pelvic version and spine balance : possible rationale for reduction. Spine (Phila Pa 1976). 2007; 32:2208–2213. PMID: 17873812.

4. Inoue H, Ohmori K, Miyasaka K. Radiographic classification of L5 isthmic spondylolisthesis as adolescent or adult vertebral slip. Spine (Phila Pa 1976). 2002; 27:831–838. PMID: 11935105.

5. Labelle H, Mac-Thiong JM, Roussouly P. Spino-pelvic sagittal balance of spondylolisthesis : a review and classification. Eur Spine J. 2011; 20(Suppl 5):641–646. PMID: 21809015.

6. Labelle H, Roussouly P, Berthonnaud E, Dimnet J, O'Brien M. The importance of spino-pelvic balance in L5-s1 developmental spondylolisthesis : a review of pertinent radiologic measurements. Spine (Phila Pa 1976). 2005; 30(6 Suppl):S27–S34. PMID: 15767882.
7. Labelle H, Roussouly P, Berthonnaud E, Transfeldt E, O'Brien M, Chopin D, et al. Spondylolisthesis, pelvic incidence, and spinopelvic balance : a correlation study. Spine (Phila Pa 1976). 2004; 29:2049–2054. PMID: 15371707.
8. Legaye J, Duval-Beaupère G, Hecquet J, Marty C. Pelvic incidence : a fundamental pelvic parameter for three-dimensional regulation of spinal sagittal curves. Eur Spine J. 1998; 7:99–103. PMID: 9629932.

9. Mac-Thiong JM, Wang Z, de Guise JA, Labelle H. Postural model of sagittal spino-pelvic alignment and its relevance for lumbosacral developmental spondylolisthesis. Spine (Phila Pa 1976). 2008; 33:2316–2325. PMID: 18827698.

10. Mehta VA, Amin A, Omeis I, Gokaslan ZL, Gottfried ON. Implications of spinopelvic alignment for the spine surgeon. Neurosurgery. 2012; 70:707–721. PMID: 21937939.

11. Osterman K, Osterman H. Experimental lumbar spondylolisthesis in growing rabbits. Clin Orthop Relat Res. 1996; 274–280. PMID: 8913172.
12. Roussouly P, Gollogly S, Berthonnaud E, Dimnet J. Classification of the normal variation in the sagittal alignment of the human lumbar spine and pelvis in the standing position. Spine (Phila Pa 1976). 2005; 30:346–353. PMID: 15682018.

13. Roussouly P, Gollogly S, Berthonnaud E, Labelle H, Weidenbaum M. Sagittal alignment of the spine and pelvis in the presence of L5-s1 isthmic lysis and low-grade spondylolisthesis. Spine (Phila Pa 1976). 2006; 31:2484–2490. PMID: 17023859.

14. Stagnara P, De Mauroy JC, Dran G, Gonon GP, Costanzo G, Dimnet J, et al. Reciprocal angulation of vertebral bodies in a sagittal plane : approach to references for the evaluation of kyphosis and lordosis. Spine (Phila Pa 1976). 1982; 7:335–342. PMID: 7135066.

15. Vialle R, Ilharreborde B, Dauzac C, Lenoir T, Rillardon L, Guigui P. Is there a sagittal imbalance of the spine in isthmic spondylolisthesis? A correlation study. Eur Spine J. 2007; 16:1641–1649. PMID: 17437136.

16. Vialle R, Levassor N, Rillardon L, Templier A, Skalli W, Guigui P. Radiographic analysis of the sagittal alignment and balance of the spine in asymptomatic subjects. J Bone Joint Surg Am. 2005; 87:260–267. PMID: 15687145.

17. Wang Z, Parent S, Mac-Thiong JM, Petit Y, Labelle H. Influence of sacral morphology in developmental spondylolisthesis. Spine (Phila Pa 1976). 2008; 33:2185–2191. PMID: 18794760.

18. Whitesides TE Jr, Horton WC, Hutton WC, Hodges L. Spondylolytic spondylolisthesis : a study of pelvic and lumbosacral parameters of possible etiologic effect in two genetically and geographically distinct groups with high occurrence. Spine. 2005; 30:S12–S21. PMID: 15767879.
19. Wiltse LL, Winter RB. Terminology and measurement of spondylolisthesis. J Bone Joint Surg Am. 1983; 65:768–772. PMID: 6863359.

Fig. 1
Schematic lumbar spine lateral images. A : Example of patients with spondylolysis. B : Example of patients without spondylolysis. SS is higher in the spondylolysis group, but STA is higher in the control group. C : Shows possible changes in sagittal balance when LL increased to maintain center of gravity and C7 plumb line behind the hips in patients with spondylolysis. SS : sacral slope, STA : sacral table angle, LL : lumbar lordosis.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download