Abstract
Objective
Cerebral hyperperfusion syndrome (CHS) is a serious complication after carotid endarterectomy (CEA). However, the prevalence of CHS has decreased as techniques have improved. This study evaluates the role of strict blood pressure (BP) control for the prevention of CHS.
Methods
All 18 patients who received CEA from February 2009 through November 2012 were retrospectively reviewed. All patients were routinely managed in an intensive care unit by a same protocol. The cerebral perfusion state was evaluated on the basis of the regional cerebral blood flow (rCBF) study by perfusion computed tomography (pCT) and mean velocity by transcranial doppler (TCD). BP was strictly controlled (<140/90 mm Hg) for 7 days. When either post-CEA hyperperfusion (>100% increase in the rCBF by pCT or in the mean velocity by TCD compared with preoperative values) or CHS was detected, BP was maintained below 120/80 mm Hg.
Results
TCD and pCT data on the patients were analyzed. Ipsilateral rCBF was significantly increased after CEA in the pCT (p=0.049). Post-CEA hyperperfusion was observed in 3 patients (18.7%) in the pCT and 2 patients (12.5%) in the TCD study. No patients developed clinical CHS for one month after CEA. Furthermore, no patients developed additional neurological deficits related to postoperative cerebrovascular complications.
Carotid endarterectomy (CEA) is a common revascularization treatment for moderate to severe stenosis of the carotid artery34). Among treatment-related complications, cerebral hyperperfusion syndrome (CHS) is a rare but major complication after CEA because it is potentially devastating5,14). The known mechanisms of CHS after CEA include diminished cerebrovascular reserve, postoperative hypertension, and hyperperfusion lasting more than several hours after CEA11,15,22,32). Another possible mechanism involves reperfusion by clamping and declamping the internal carotid artery, which produces oxygen-derived free radicals10,31). CHS is traditionally characterized by an ipsilateral throbbing headache, eye and face pain, seizures, and focal neurological deficits related to cerebral edema or intracerebral hemorrhage (ICH)3,13,26,29,30,33). The reported prevalence of CHS, regardless of revascularization method, varies from 0% through 18.9%1,6,11-13,18-22,24,25,33,34). Because the diagnosis of CHS is based on several nonspecific symptoms and the diagnostic definition is not strict, patients may sometimes be misdiagnosed.
Possible risk factors of CHS include hypertension, diabetes mellitus, old age (≥72 years), contralateral carotid occlusion, and use of anticoagulant or antiplatelet therapy34). Due to improvements in surgical skill and postoperative care, the prevalence of CHS appears to have decreased. The authors of two recent studies reported that no patients had CHS after CEA or carotid stenting13,20). Other investigators have suggested that CHS is a preventable cause of perioperative stroke following CEA5). The aim of this study was to evaluate the role of current intensive care protocols with strict blood pressure (BP) control for CEA to prevent CHS.
We retrospectively reviewed the records of all patients receiving CEA at our hospital from February 2009 through November 2012. CEA was indicated due to transient ischemic attack (TIA), mild cerebral infarction with ipsilateral carotid stenosis (>60%), or high-grade carotid stenosis (>60%) without associated symptoms17). Cerebral blood flow (CBF) was measured as regional cerebral blood flow (rCBF) by perfusion computed tomography (pCT) and mean velocity (Vmean) by transcranial doppler (TCD) preoperatively (within 1 month before CEA) and postoperatively (within 1 week after CEA). A total of 18 patients received CEA at our institution over the 4-year review period. Among the patients, 16 patients who were fully evaluated were enrolled in this study. Patient demographics, clinical presentation, stenosis severity, radiological studies, and operative variables were reviewed for each case.
Post-CEA hyperperfusion (PHP) has been defined by 2 standards : 1) >100% increase in rCBF in the anterior cerebral artery (ACA) territories compared with the preoperative value by perfusion CT2,13); and 2) >100% increase of the preoperative Vmean in the middle cerebral artery (MCA) without clinical symptoms by TCD25). The definition for CHS requires the fulfillment of the following four criteria5) : 1) occurrence within 30 days after CEA; 2) evidence of hyperperfusion on TCD, pCT imaging, or systolic BP >180 mm Hg; 3) clinical features such as new headache, seizure, hemiparesis, Glasgow Coma Scale <15, or radiological features such as cerebral edema or ICH; and 4) no evidence of new cerebral ischemia, postoperative carotid occlusion, and metabolic or pharmacologic causes.
CEA was performed using the traditional method as described in a previous study17). During CEA, a neurologist monitored the electroencephalograms of all patients. The carotid artery was closed primarily without a graft. Shunts were not typically used during the CEA. If the clamping time passes above 25 minutes, a silicone shunt was placed. After CEA, all patients were transferred directly to an intensive care unit (ICU) because strict BP control is sometimes difficult when patients come out of anesthesia.
The postoperative management protocol is shown in Fig. 1. All patients were managed routinely under continuous sedation for several hours after CEA in the ICU. BP was monitored continuously and strictly controlled (<140/90 mm Hg). Anti-hypertensive treatment consisted of intravenous labetalol (first choice) continuous infusion and/or bolus infusion. If BP was not controlled, oral clonidine was added. If BP was within the required limits, intravenous anti-hypertensive treatment was tapered as soon as possible and an oral beta-blocker (labetalol or metoprolol) was started. When CHS was detected, postoperative systemic BP was more stringently controlled to maintain it below 120/80 mm Hg. The cerebral perfusion state was routinely evaluated by pCT at postoperative 2 days and TCD at postoperative 0, 2, and 7 days after CEA or on detecting CHS.
SPSS (version 20.0, 2011; SPSS Inc., Chicago, IL, USA) was used for statistical analysis. The preoperative and postoperative CBFs were compared using a paired t test. Differences with a p value of less than 0.05 were considered statistically significant. The values are presented as the mean±standard deviation.
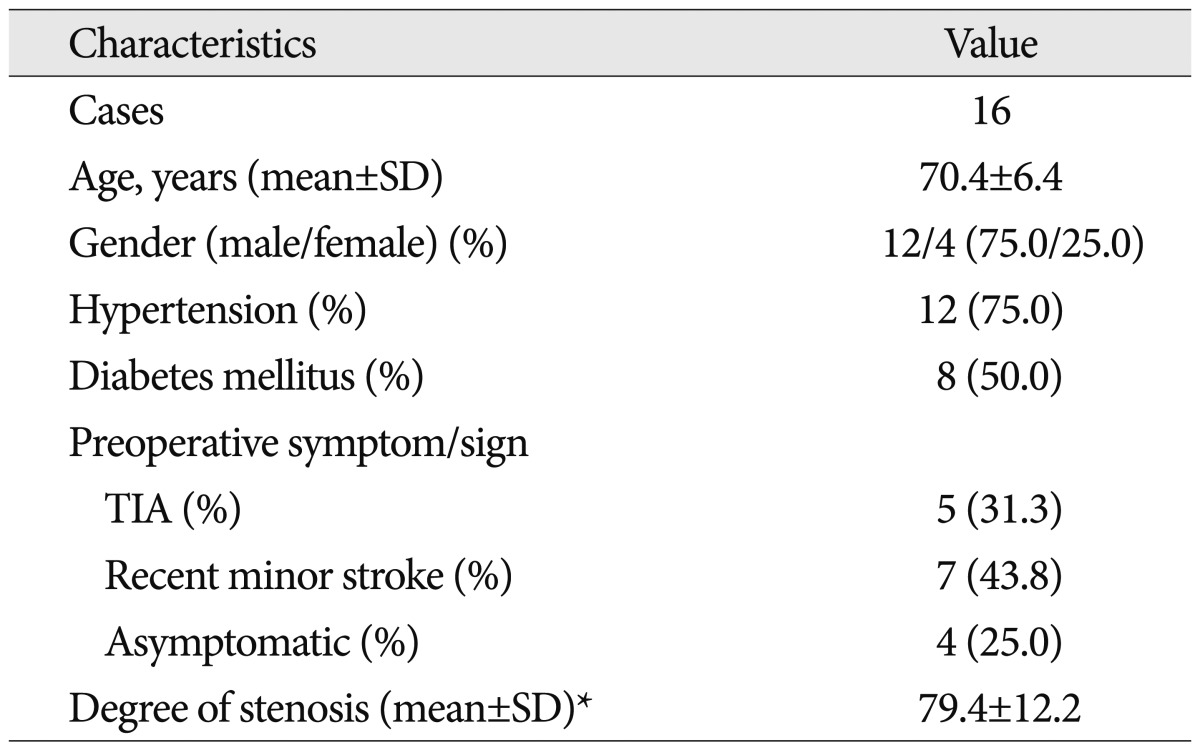
A total of 16 patients was analyzed in this study. The patient demographics are summarized in Table 1. The patient population consisted of 12 men and 4 women ranging in age from 59 to 81 years (mean±standard deviation, 70.4±6.4 years). Severity of ipsilateral internal carotid artery (ICA) stenosis was ranged from 60% to 99% (mean±standard deviation, 79.4±12.2%). Comorbidities associated with carotid disease, such as hypertension (75.0%) and diabetes mellitus (50.0%), were common among the patients. The indications for CEA were asymptomatic ICA stenosis (4 patients, 25.0%), recent minor stroke (7 patients, 43.8%), and TIA (5 patients, 31.3%). TIA presented as hemiparesis in 3 patients, amaurosis fugax in 1 patient, and hemiparesthesia in 1 patient.
Post-CEA Vmean change on the MCA territory by the TCD was shown in Fig. 2. Among 16 patients, 2 patients (12.5%) developed post-CEA hyperperfusion by the TCD. One patient was a 68-year-old man and had a history of transient left hemiparesis. His right ICA stenosis was 70.0%. He has taken antihypertensive medications. Post-CEA hyperperfusion was detected by the TCD on the second postoperative day and was normalized on the seventh postoperative day. The other patient was a 76-year-old man and had left hemiparesis. His right ICA stenosis was 86.2%. He had no underlying disease. Post-CEA hyperperfusion was detected by the TCD on the seventh postoperative day. These 2 patients had uneventful postoperative courses and did not developed clinical CHS for one month after CEA.
Post-CEA mean rCBF change on the ACA territory was measured by pCT in Fig. 3. Bilateral ACA blood flows increased. The mean rCBF on the ipsilateral side increased from 45.3 to 70.1 mL/100 g/min after CEA, which has a statistical significance (p=0.049). The mean rCBF on the contralateral side increased from 50.3 to 70.1 mL/100 g/min after CEA (p=0.221). PHP was observed in 3 cases (18.7%) on the pCT result of the 16 patients. Among 3 patients, 68-year-old and 76-year-old men who also showed PHP by the TCD presented CBF increment as 183.5% and 150.8%, respectively. The other patient is a 65-year-old man and had right amaurosis fugax. His right ICA stenosis was 84%. Vmean of the patient was not met the criteria for PHP. No patients developed additional neurological deficits related to postoperative cerebrovascular complications. No morbidity or mortality was observed in the present series.
Many investigators have analyzed the prevalence and risk factors of CHS. Almost all studies suggested that strict BP control was the most important and was a mainstay of post-CEA management7,9,18,23,25,27,34). However, we do not know how to best manage the patients underwent CEA : the targets for blood pressure levels after CEA (to prevent postoperative ischemic stroke risk and at the same time avoid cerebral hyperperfusion) are not well defined7,28). The meta-analysis study reported that there is no published case of CHS below systolic BP of 135 mm Hg5). In the literature review, 2 studies used a guideline as systolic BP <160 mm Hg and showed 5.0% of CHS in Table 219,25). Three studies complied with BP guideline as systolic BP <140 mm Hg and systolic BP was more stringently controlled to lower than 120 mm Hg when post-CEA hyperperfusion was detected1,13,33). The CHS incidence of these studies is 1.9%, 0.5%, and 0%. Our study also complied with this BP guideline and accomplished 0% of CHS.
In order to control increased BP, one book suggested that nicardipine (calcium antagonists) was the agent of choice8). However, van Mook et al.34) insisted that many drugs commonly used for treatment of hypertension such as calcium antagonists and direct vasodilators (for example, nitroprusside or glycerol trinitrate) are contraindicated on theoretical ground. They suggested beta blocker such as labetalol for control PHP34). Another paper addressed nitroglycerin (vasodilator) was used without complication1). Labetalol is usually used in many studies. Efficacy and safety of other medications are need to further studies.
In terms of postoperative care at an ICU, the current American Heart Association guidelines4) for CEA state that patients who undergo CEA should use ICU resources as recommended in major surgical texts. We continuously monitored and controlled BP immediately using intravenous beta blockers for 2 days after CEA in the ICU, followed by oral or intravenous antihypertensive medications in a general ward. ICU care can also offer that medical attendees are checking the CHS under constant surveillance.
Three limitations should be addressed regarding the present study. First, the present investigation was retrospective and not fully controlled, as other medications, including antiplatelet therapy or anticoagulants, were occasionally used. Therefore, the results of the present study are not definitive. Second, the CBFs in this study were analyzed according to the classic region of interest (ROI) method, in which ROIs are drawn manually as a way to solve visual assessment. However, this method has been shown to suffer from significant intra-observer and inter-observer variation16). Finally, vascular reactivity is also important to PHP. However, single-photon emission computed tomography was not available in our institute during the study period and this study could not contain the result.
Rigorous blood pressure control remains the mainstay of treatment. Intensive care with strict BP control (<140/90 mm Hg) achieved a low prevalence of post-CEA hyperperfusion and prevented post-CEA CHS. This study suggests that intensive care with strict BP control can prevent the prevalence of post-CEA CHS.
References
1. Abou-Chebl A, Reginelli J, Bajzer CT, Yadav JS. Intensive treatment of hypertension decreases the risk of hyperperfusion and intracerebral hemorrhage following carotid artery stenting. Catheter Cardiovasc Interv. 2007; 69:690–696. PMID: 17377975.

2. Adhiyaman V, Alexander S. Cerebral hyperperfusion syndrome following carotid endarterectomy. QJM. 2007; 100:239–244. PMID: 17307751.

3. Bernstein M, Fleming JF, Deck JH. Cerebral hyperperfusion after carotid endarterectomy : a cause of cerebral hemorrhage. Neurosurgery. 1984; 15:50–56. PMID: 6472594.

4. Biller J, Feinberg WM, Castaldo JE, Whittemore AD, Harbaugh RE, Dempsey RJ, et al. Guidelines for carotid endarterectomy : a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 1998; 29:554–562. PMID: 9480580.
5. Bouri S, Thapar A, Shalhoub J, Jayasooriya G, Fernando A, Franklin IJ, et al. Hypertension and the post-carotid endarterectomy cerebral hyperperfusion syndrome. Eur J Vasc Endovasc Surg. 2011; 41:229–237. PMID: 21131217.

6. Coutts SB, Hill MD, Hu WY. Hyperperfusion syndrome : toward a stricter definition. Neurosurgery. 2003; 53:1053–1058. discussion 1058-1060. PMID: 14580271.
7. De Rango P. Cerebral hyperperfusion syndrome : the dark side of carotid endarterectomy. Eur J Vasc Endovasc Surg. 2012; 43:377. PMID: 22289610.

8. Greenberg MS. Handbook of Neurosurgery. ed 7. Florida: Thieme;2010. p. 1152.
9. Henderson RD, Phan TG, Piepgras DG, Wijdicks EF. Mechanisms of intracerebral hemorrhage after carotid endarterectomy. J Neurosurg. 2001; 95:964–969. PMID: 11765840.

10. Holm J, Nilsson U, Waters N, Waters S, Jonsson O. Production of free radicals measured by spin trapping during operations for stenosis of the carotid artery. Eur J Surg. 2001; 167:4–9. PMID: 11213819.
11. Hosoda K, Kawaguchi T, Shibata Y, Kamei M, Kidoguchi K, Koyama J, et al. Cerebral vasoreactivity and internal carotid artery flow help to identify patients at risk for hyperperfusion after carotid endarterectomy. Stroke. 2001; 32:1567–1573. PMID: 11441203.

12. Jørgensen LG, Schroeder TV. Defective cerebrovascular autoregulation after carotid endarterectomy. Eur J Vasc Surg. 1993; 7:370–379. PMID: 8359291.

13. Kawamata T, Okada Y, Kawashima A, Yoneyama T, Yamaguchi K, Ono Y, et al. Postcarotid endarterectomy cerebral hyperperfusion can be prevented by minimizing intraoperative cerebral ischemia and strict postoperative blood pressure control under continuous sedation. Neurosurgery. 2009; 64:447–453. discussion 453-454. PMID: 19240606.

14. Kim DE, Choi SM, Yoon W, Kim BC. Hyperperfusion syndrome after carotid stent-supported angioplasty in patients with autonomic dysfunction. J Korean Neurosurg Soc. 2012; 52:476–479. PMID: 23323169.

15. Komoribayashi N, Ogasawara K, Kobayashi M, Saitoh H, Terasaki K, Inoue T, et al. Cerebral hyperperfusion after carotid endarterectomy is associated with preoperative hemodynamic impairment and intraoperative cerebral ischemia. J Cereb Blood Flow Metab. 2006; 26:878–884. PMID: 16280980.

16. Lagreze HL, Levine RL, Sunderland JS, Nickles RJ. Pitfalls of regional cerebral blood flow analysis in cerebrovascular disease. Clin Nucl Med. 1988; 13:197–201. PMID: 3260164.

17. Lee CH, Jung YS, Yang HJ, Son YJ, Lee SH. An innovative method for detecting surgical errors using indocyanine green angiography during carotid endarterectomy : a preliminary investigation. Acta Neurochir (Wien). 2012; 154:67–73. discussion 73. PMID: 22068716.

18. Maas MB, Kwolek CJ, Hirsch JA, Jaff MR, Rordorf GA. Clinical risk predictors for cerebral hyperperfusion syndrome after carotid endarterectomy. J Neurol Neurosurg Psychiatry. 2013; 84:569–572. PMID: 23243262.

19. Meyers PM, Higashida RT, Phatouros CC, Malek AM, Lempert TE, Dowd CF, et al. Cerebral hyperperfusion syndrome after percutaneous transluminal stenting of the craniocervical arteries. Neurosurgery. 2000; 47:335–343. discussion 343-345. PMID: 10942006.

20. Miyamoto N, Naito I, Takatama S, Shimizu T, Iwai T, Shimaguchi H. Urgent stenting for patients with acute stroke due to atherosclerotic occlusive lesions of the cervical internal carotid artery. Neurol Med Chir (Tokyo). 2008; 48:49–55. discussion 55-56. PMID: 18296872.

21. Morrish W, Grahovac S, Douen A, Cheung G, Hu W, Farb R, et al. Intracranial hemorrhage after stenting and angioplasty of extracranial carotid stenosis. AJNR Am J Neuroradiol. 2000; 21:1911–1916. PMID: 11110546.
22. Naylor AR, Evans J, Thompson MM, London NJ, Abbott RJ, Cherryman G, et al. Seizures after carotid endarterectomy : hyperperfusion, dysautoregulation or hypertensive encephalopathy? Eur J Vasc Endovasc Surg. 2003; 26:39–44. PMID: 12819646.

23. Ogasawara K, Sakai N, Kuroiwa T, Hosoda K, Iihara K, Toyoda K, et al. Intracranial hemorrhage associated with cerebral hyperperfusion syndrome following carotid endarterectomy and carotid artery stenting : retrospective review of 4494 patients. J Neurosurg. 2007; 107:1130–1136. PMID: 18077950.

24. Ogasawara K, Yukawa H, Kobayashi M, Mikami C, Konno H, Terasaki K, et al. Prediction and monitoring of cerebral hyperperfusion after carotid endarterectomy by using single-photon emission computerized tomography scanning. J Neurosurg. 2003; 99:504–510. PMID: 12959438.

25. Pennekamp CW, Tromp SC, Ackerstaff RG, Bots ML, Immink RV, Spiering W, et al. Prediction of cerebral hyperperfusion after carotid endarterectomy with transcranial Doppler. Eur J Vasc Endovasc Surg. 2012; 43:371–376. PMID: 22264422.

26. Reigel MM, Hollier LH, Sundt TM Jr, Piepgras DG, Sharbrough FW, Cherry KJ. Cerebral hyperperfusion syndrome : a cause of neurologic dysfunction after carotid endarterectomy. J Vasc Surg. 1987; 5:628–634. PMID: 3560356.

27. Ricotta JJ, Aburahma A, Ascher E, Eskandari M, Faries P, Lal BK. Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease. J Vasc Surg. 2011; 54:e1–e31. PMID: 21889701.

28. Ricotta JJ, Aburahma A, Ascher E, Eskandari M, Faries P, Lal BK. Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease : executive summary. J Vasc Surg. 2011; 54:832–836. PMID: 21889705.

29. Schroeder T, Sillesen H, Sørensen O, Engell HC. Cerebral hyperperfusion following carotid endarterectomy. J Neurosurg. 1987; 66:824–829. PMID: 3572512.

30. Solomon RA, Loftus CM, Quest DO, Correll JW. Incidence and etiology of intracerebral hemorrhage following carotid endarterectomy. J Neurosurg. 1986; 64:29–34. PMID: 3941347.

31. Soong CV, Young IS, Hood JM, Rowlands BJ, Trimble ER, Barros D'Sa AA. The generation of byproducts of lipid peroxidation following carotid endarterectomy. Eur J Vasc Endovasc Surg. 1996; 12:455–458. PMID: 8980437.

32. Sundt TM Jr, Sharbrough FW, Piepgras DG, Kearns TP, Messick JM Jr, O'Fallon WM. Correlation of cerebral blood flow and electroencephalographic changes during carotid endarterectomy : with results of surgery and hemodynamics of cerebral ischemia. Mayo Clin Proc. 1981; 56:533–543. PMID: 7266064.
33. Terada T, Tsuura M, Matsumoto H, Masuo O, Tsumoto T, Yamaga H, et al. Hemorrhagic complications after endovascular therapy for atherosclerotic intracranial arterial stenoses. Neurosurgery. 2006; 59:310–318. discussion 310-318. PMID: 16823323.

34. van Mook WN, Rennenberg RJ, Schurink GW, van Oostenbrugge RJ, Mess WH, Hofman PA, et al. Cerebral hyperperfusion syndrome. Lancet Neurol. 2005; 4:877–888. PMID: 16297845.

Fig. 1
Algorithm for the treatment of patients after CEA. CEA : carotid endarterectomy, ICU : intensive care unit, BP : blood pressure, TCD : transcranial doppler, CHS : cerebral hyperperfusion syndrome, CT : computed tomography.
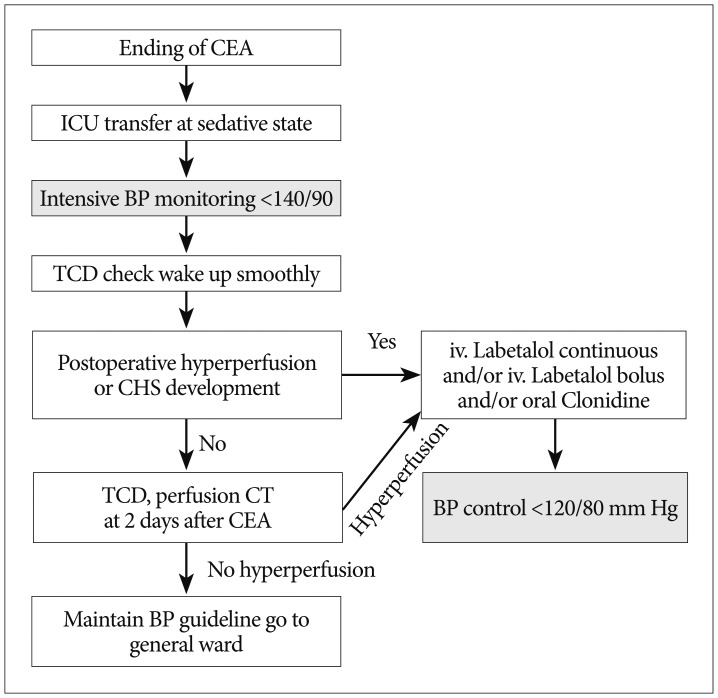
Fig. 2
Typical pattern of changes in Vmean after CEA by TCD. TCD shows the development of PHP in 2 patients (asterisks). CEA : carotid endarterectomy, TCD : transcranial doppler, PHP : post-CEA hyperperfusion.

Fig. 3
Comparison of preoperative and postoperative CBF status; mean CBF at the ipsilateral side increased by 54.8%. The CBF increase on the ipsilateral side to CEA is statistically significant (p=0.049). The CBF on the contralateral side also increased by 31.2% after CEA (p=0.221).
*Statistically significant difference between preoperative and postoperative CBF. CBF : cerebral blood flow, CEA : carotid endarterectomy.
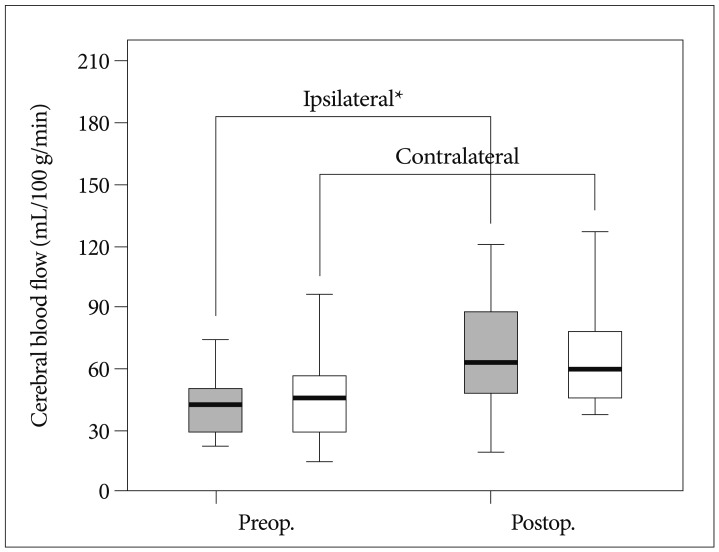
Table 2
The relation with blood pressure control and cerebral hyperperfusion syndrome
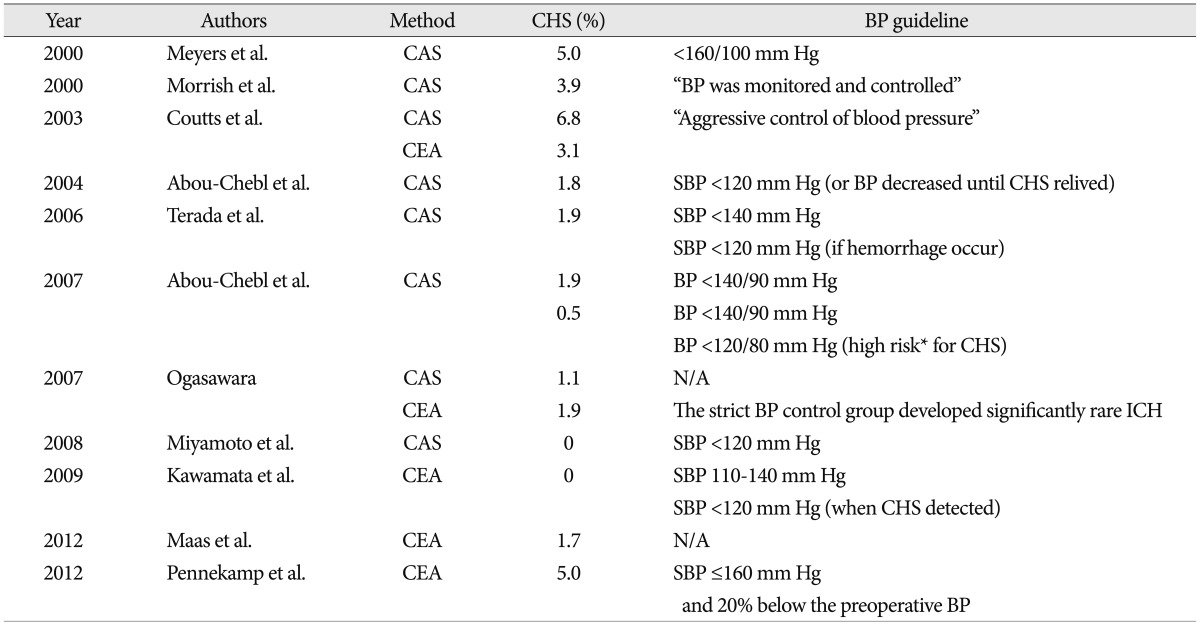
*High-risk criteria included the presence of hypertension at baseline, a treated carotid stenosis of >90%, poor collateral blood flow, or contralateral carotid stenosis >80%. CHS : cerebral hyperperfusion syndrome, BP : blood pressure, CAS : carotid artery stenting, N/A : not addressed, CEA : carotid endarterectomy, SBP : systolic blood pressure, ICH : intracerebral hemorrhage




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download