Abstract
Here, we report a rare case of an anaplastic astrocytoma masquerading as a hypertensive basal ganglia hemorrhage. A 69-year-old woman who had been under medical management for hypertension during the past 3 years suddenly developed right hemiparesis with dysarthria. Brain computed tomography (CT) scans with contrast and CT angiograms revealed an intracerebral hemorrhage (ICH) in the left basal ganglia, without an underlying lesion. She was treated conservatively, but underwent a ventriculoperitoneal shunt operation 3 months after the initial attack due to deteriorated mental status and chronic hydrocephalus. Three months later, her mental status deteriorated further. Magnetic resonance imaging (MRI) with gadolinium demonstrated an irregular enhanced mass in which the previous hemorrhage occurred. The final histological diagnosis which made by stereotactic biopsy was an anaplastic astrocytoma. In the present case, the diagnosis of a high grade glioma was delayed due to tumor bleeding mimicking hypertensive ICH. Thus, a careful review of neuroradiological images including MRI with a suspicion of tumor bleeding is needed even in the patients with past medical history of hypertension.
Hypertensive vasculopathy is the most common etiology for spontaneous intracerebral hemorrhage (ICH). Additional causes of nontraumatic ICH include hemorrhagic infarction, septic embolism, brain tumors, bleeding disorders, anticoagulants, vasculopathy (including moyamoya disease and arteriovenous malformations), and drugs. Among these, brain tumors are a relatively rare but important cause of ICH5). Hemorrhage rates in patients with intracranial neoplasms range from 2% to 5%2). However, apoplectic onset of hemorrhage from a silent brain tumor is even more uncommon. Only 0.6% of brain tumors abruptly develop hemorrhage, without prior symptoms9). Here, we report a rare case of anaplastic astrocytoma with tumor bleeding mimicking a spontaneous hypertensive intracerebral hemorrhage.
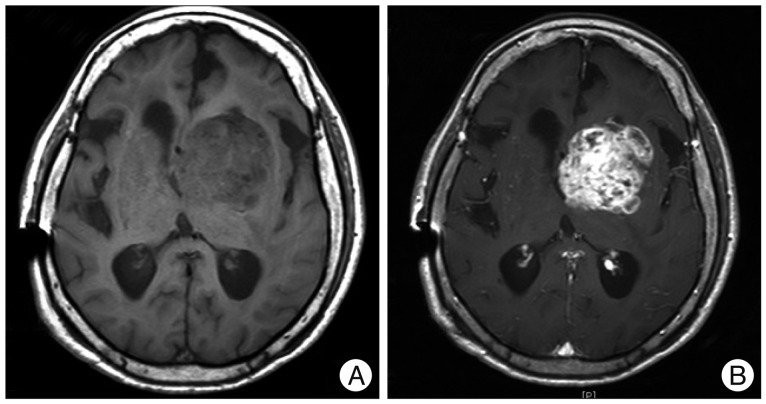
A 69-year-old woman visited our hospital presenting with nausea, vomiting, dysarthria, and right hemiparesis. The patient had been under medical treatment for hypertension for the previous 3 years. Brain computed tomography (CT) scans with contrast enhancement and CT angiograms demonstrated an acute intracerebral hematoma in the left basal ganglia, including the caudate nucleus, accompanied by a small amount of intraventricular hemorrhage, without any underlying enhanced lesion or vascular pathologies (Fig. 1A, B). The patient was treated conservatively with anti-hypertensive drugs under a diagnosis of hypertensive basal ganglia ICH. Additional physical rehabilitation was performed, and the patient was discharged with improved motor function and mental status 6 weeks after admission. During follow-up as an outpatient, the patient was re-admitted for physical malaise and deteriorated mental status 6 weeks after discharge. Brain CT scans demonstrated asymmetric ventriculomegaly and low periventricular density (Fig. 1C, D). Her mental status was temporarily improved and the ventricle size was decreased following a ventriculoperitoneal (VP) shunt operation. Three months after VP shunt placement, the patient's mental status deteriorated further and quadriparesis was noted. Brain magnetic resonance imaging (MRI) with gadolinium enhancement revealed an irregular, enhanced, round mass with multiple necrotic foci in the left basal ganglia, where the previous hemorrhage had occurred (Fig. 2). Navigation-assisted biopsy was performed, revealing an anaplastic astrocytoma (WHO grade III). The patient underwent radiotherapy, and repeat MR imaging revealed non-progression of the tumor (data not shown). However, she has been bed-ridden during 6 months of follow-up.
Intratumoral hemorrhage is thought to originate from abnormal newborn vessels that traverse necrotic areas9) or from tumoral invasion of large vessels12), leading to thinning and rupture of the vessels walls. Another potential mechanism would be relatively weak tumor vessels, which are not well invested with a glial meshwork; this may contribute to reduced resistance to the shearing forces of the brain1). Endothelial proliferation with subsequent obliteration of the lumen or presence of intratumoral arteriovenous fistulae are alternate explanations for intratumoral bleeding7). Thus, hemostasis often cannot be easily achieved at hematoma removal; this finding indicates the possibility of brain tumors as a cause of bleeding10).
There have been known risk factors of intratumoral hemorrhage. Hemorrhage more often develops in malignant tumors such as glioblastoma and metastatic brain tumors4,9,11). The incidence of tumor bleeding in malignant astrocytoma in one study was 6%4) while that in glioblastoma and metastatic brain tumors were 6.5-8% and 7-9%, respectively4,9). Among benign neuroepithelial tumors, the incidence of hemorrhage from mixed glioma and oligodendroglioma was much higher than the other tumors4,9). On the other hand, pituitary adenoma and meningiomas have the high risk for developing intratumoral hemorrhage among benign non-neuroepithelial tumors11). The location of bleeding depends on the different site of brain tumor even though intratumoral hemorrhage usually develops in the atypical location of hypertensive intracerebral hemorrhage and the patients often have no history of hypertension11,12).
Radiological studies with contrast material usually distinguish tumors from hemorrhage, as the border between the tumors and hemorrhage is usually clear6). In contrast, if the tumors are compressed by a large hemorrhage, or the border between the tumors and hemorrhage is unclear, intratumoral hemorrhage may be indistinguishable from spontaneous ICH, even though contrast material is used3). Thus, a CT with contrast cannot exclude underlying pathologies that may cause ICH, especially if the patient has a history of hypertension, and the location is typical for hypertensive ICH. MRI with gadolinium in the early follow-up period would likely have lead to earlier detection of the tumors in the present case. However, Inamasu et al.3) suggested that in terms of cost effectiveness, it is controversial to have every patient presenting with typical hypertensive ICH undergo MRI with gadolinium to rule out intratumoral bleeding.
Histopathological examination of hematoma specimens with brain parenchyma may have detected the brain tumor, if hematoma evacuation or aspiration surgery had been performed in the current case. However, the relatively small volume of hematoma and patient's age led us to choose conservative treatments, rather than surgical options. It is controversial to undergo surgical treatments for histological diagnosis in typical hypertensive small ICH, though potential surgical modalities include stereotactic hematoma aspiration or endoscopic hematoma evacuation. However, if surgical treatment is indicated, a considerable hematoma specimen with adjacent brain parenchyma would be preferred to rule out underlying pathologies through histological examination.
Interestingly, normal initial neuroimaging including MRI is not uncommon among patients with malignant primary brain tumors. Thaler et al.8) reported that among 193 patients with malignant primary brain tumors, initial imaging preceding diagnosis with a short interval was normal in nine patients and abnormal but non-diagnositic in an additional eight patients. They conclude that dramatic, rapid tumor growth is possible8). Thus, in our case, it cannot be excluded that basal ganglia hemorrhage was a hypertensive hemorrhage which was not related with brain tumor, and then anaplastic astrocytoma rapidly developed in the site of hemorrhage.
In the present case, there was an opportunity to detect the underlying high-grade glioma during outpatient follow-up. The patient suffered from physical malaise and a deteriorated mental status 6 weeks after discharge. However, only a CT was performed as the etiology of the ICH was presumed to be hypertension. A follow-up CT revealed asymmetric ventriculomegaly and periventricular edema, with shunt operation performed without additional radiological studies. As a result, the final diagnosis of the tumor was delayed. However, in retrospect, asymmetric ventriculomegaly suggests an underlying pathology in the region of the pre-existing hemorrhage. Thus, follow-up MRI in the chronic stage of ICH seems to be necessary for detecting underlying pathologies masquerading as hemorrhage if the patient undergoes subtle neurological deterioration during follow-up in order to avoid diagnostic delay, even in cases typical for hypertensive hemorrhage.
The present case indicates that some brain tumors, masquerading as a hypertensive ICH, may be difficult to diagnosis in the acute phase. A diagnostic delay for highly malignant tumors leads to progression without proper treatment and resultant poor outcomes. Thus, although the morphology and location of the hematoma and patient medical history are typical for a hypertensive ICH, brain tumors should be suspected as a cause of spontaneous hemorrhage. If needed, aggressive histological investigation at hematoma evacuation and MRI follow-up should be considered to avoid hidden, underlying pathologies.
Acknowledgements
This study was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A1203920300).
References
1. Can SM, Aydin Y, Turkmenoglu O, Aydin F, Ziyal I. Giant cell glioblastoma manifesting as traumatic intracerebral hemorrhage--case report. Neurol Med Chir (Tokyo). 2002; 42:568–571. PMID: 12513031.

2. Cemil B, Tun K, Polat O, Ozen O, Kaptanoglu E. Glioblastoma multiforme mimicking arteriovenous malformation. Turk Neurosurg. 2009; 19:433–436. PMID: 19847768.
3. Inamasu J, Kuramae T, Nakatsukasa M. Glioblastoma masquerading as a hypertensive putaminal hemorrhage : a diagnostic pitfall. Neurol Med Chir (Tokyo). 2009; 49:427–429. PMID: 19779291.

4. Kondziolka D, Bernstein M, Resch L, Tator CH, Fleming JF, Vanderlinden RG, et al. Significance of hemorrhage into brain tumors : clinicopathological study. J Neurosurg. 1987; 67:852–857. PMID: 3316531.
5. Morgenstern LB, Frankowski RF. Brain tumor masquerading as stroke. J Neurooncol. 1999; 44:47–52. PMID: 10582668.
6. Nakayama Y, Tanaka A, Yoshinaga S, Ueno Y. [Indications for surgery to determine the etiology of subcortical hemorrhage]. No Shinkei Geka. 1998; 26:1067–1074. PMID: 9883445.
7. Schrader B, Barth H, Lang EW, Buhl R, Hugo HH, Biederer J, et al. Spontaneous intracranial haematomas caused by neoplasms. Acta Neurochir (Wien). 2000; 142:979–985. PMID: 11086806.

8. Thaler PB, Li JY, Isakov Y, Black KS, Schulder M, Demopoulos A. Normal or non-diagnostic neuroimaging studies prior to the detection of malignant primary brain tumors. J Clin Neurosci. 2012; 19:411–414. PMID: 22277560.

9. Wakai S, Yamakawa K, Manaka S, Takakura K. Spontaneous intracranial hemorrhage caused by brain tumor : its incidence and clinical significance. Neurosurgery. 1982; 10:437–444. PMID: 7099393.
10. Watanabe K, Wakai S, Okuhata S. Gliomas presenting with basal ganglionic haemorrhage. Report of two cases. Acta Neurochir (Wien). 1997; 139:787–788. PMID: 9309296.

11. Yuguang L, Meng L, Shugan Z, Yuquan J, Gang L, Xingang L, et al. Intracranial tumoural haemorrhage--a report of 58 cases. J Clin Neurosci. 2002; 9:637–639. PMID: 12604273.
12. Zimmerman RA, Bilaniuk LT. Computed tomography of acute intratumoral hemorrhage. Radiology. 1980; 135:355–359. PMID: 7367626.

Fig. 1
Initial brain computed tomography (CT) demonstrates an intracerebral hemorrhage at the left basal ganglia, including the caudate nucleus (A). CT scan with contrast reveals no additional information about underlying pathology (B). Six weeks after discharge, follow-up CT scans demonstrates asymmetric ventriculomegaly and periventricular low density (C and D).
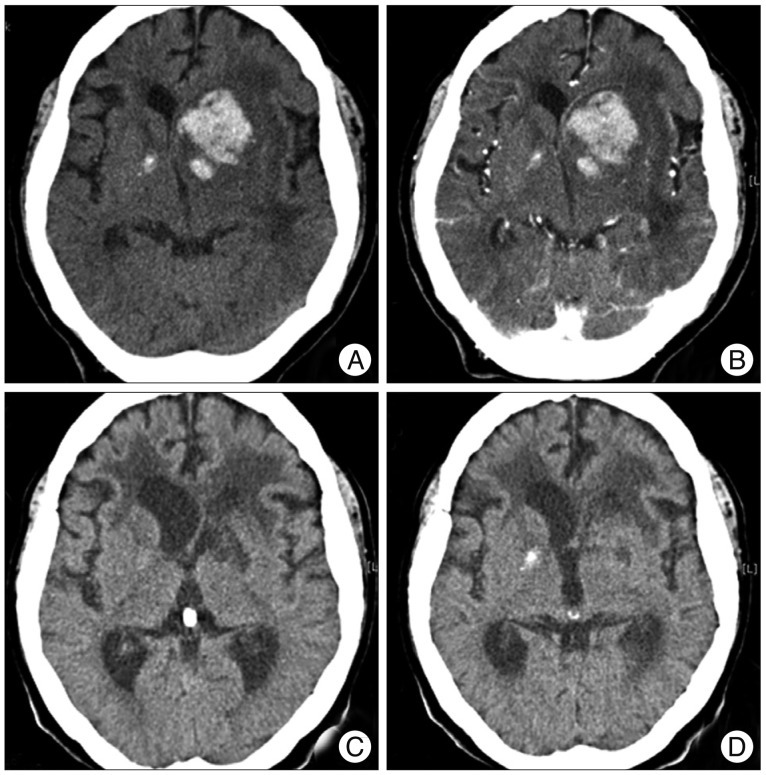
Fig. 2
Three months after the shunt operation, T1-weighted magnetic resonance images (A) with gadolinium (B) demonstrates an irregular, enhanced, round mass with multiple necrotic foci in the left basal ganglia, where the previous hemorrhage had occurred. A previous hematoma cavity is not visualized.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download