Abstract
The small pituitary mass was incidentally found in 40-years-old women with renal cell carcinoma. The endocrinological and ophthalmological evaluation revealed no deficit and the short-term follow-up was recommended. In 6 months later, the visual disturbance was reported and the size of mass was increased. The tumor was removed totally via the trans-sphenoid approach. The post-operative endocrinological insufficiency was not noticed. During one year of follow-up period, there was no evidence of recurrence without adjuvant radiotherapy. The clinical features of pituitary metastasis from renal cell carcinoma were similar to those of pituitary adenoma. The possibility of pituitary metastasis should be kept in mind in patients with sellar mass and renal cell carcinoma.
Metastatic tumors in the pituitary gland are uncommon and difficult to diagnose because most are small and clinically silent9). Only 6.8% of pituitary metastases are symptomatic, although a large autopsy series reported a 3.6% incidence of pituitary metastasis7,9). The original sites of malignancies in pituitary metastasis do not differ greatly from those in cerebral parenchymal metastasis. In pituitary metastasis, the most common primary malignancies are breast cancer in females and lung cancer in males, and other systemic metastases are often associated1,5). However, to the best of our knowledge, pituitary metastasis from renal cell carcinoma has been was rarely reported, and only 25 cases have been published in the literature1,2,4). We present a case of pituitary metastasis from renal cell carcinoma.
A 40-year-old woman presented with mild headache. Three years prior, she had undergone a left radical nephrectomy for renal cell carcinoma. Video-assisted thoracic surgery was performed for metastatic lymph node excision and left lower lobe superior segmentectomy. Additional procedures included the excision of a retroperitoneal lymph node metastasis and thoracotomy for lymph node dissection along the descending aorta. She received chemotherapy for multiple metastases, including the adrenal gland, psoas muscle and mediastinum. During the follow-up after chemotherapy, she complained of intermittent headache. Magnetic resonance (MR) imaging demonstrated a 13 mm sellar mass with suprasellar extension without optic nerve compression (Fig. 1). The pituitary stalk was deviated to the left side. An endocrinological evaluation revealed hyperprolactinemia as follows : serum prolactin, 89.5 ng/mL [reference range (RR) : 0-25 ng/mL]; free T4, 0.75 ng/dL (RR : 0.70-1.80 ng/dL); TSH, 1.73 uIU/mL (RR : 0.4-4.1 uIU/m); ACTH at 8 am, 52 pg/mL (RR : 0-60 pg/mL); Cortisol at 8 am, 7.2 ug/dL (RR : 5-25 ug/dL); LH, 6.7 mIU (RR : 1-12 mIU); and FSH, 11.5 mIU (RR : 2-13 mIU). 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) showed no uptake in the sellar area, and increased uptake in metastatic lesions in other sites (Fig. 1). She did not complain of the visual symptoms, and Goldmann perimetry revealed no visual field defects. A careful history revealed that she had experienced galactorrhea and amenorrhea, which continued for 5 months and resolved without management 9 years ago prior to her current presentation. With these clinical backgrounds, a presumptive diagnosis of pituitary adenoma was made and regular follow-up was recommended because of the relatively rare incidence of pituitary metastasis.
Six months after the first visit, her vision worsened. Snellen visual acuity without correction at a 5 m distance in 200 lux of light showed a marked decrease in visual acuity from 1.0/1.0 to 1.0/0.4. Goldmann perimetry revealed superior temporal quadrantanopsia of the left eye. The follow-up MR imaging showed that the mass from 13 mm to 23 mm, and compression of the optic apparatus was observed (Fig. 2). The cocktail test showed hyperprolacinemia (prolactin, 74.4 ng/mL) and hypocortisolism (peak cortisol, 11.9 ug/dL). The other pituitary axes were intact, and there were no symptoms suggesting diabetes insipidus.
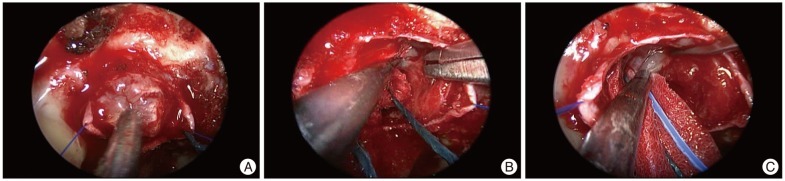
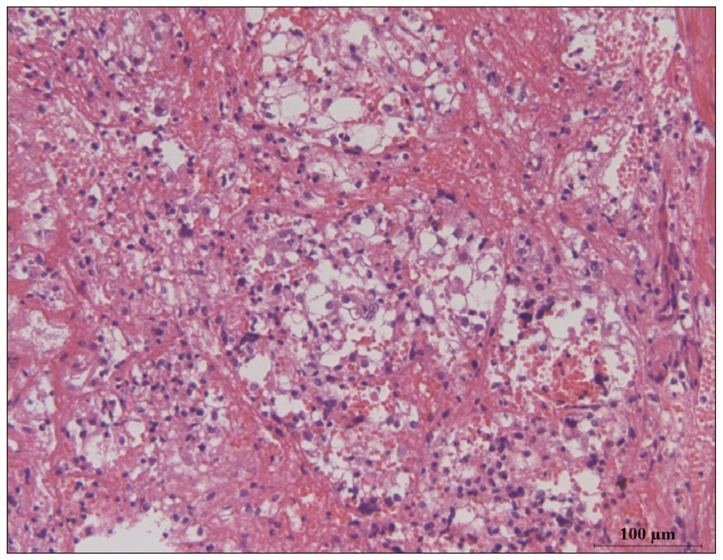
The patient underwent trans-sphenoid surgery to obtain a pathologic diagnosis and decompress the optic apparatus. The sellar floor was very thin and showed a mottled appearance. The tumor was gray and hypervascular (Fig. 3). Its hard consistency and the distinct demarcation helped to easily dissect it from the pituitary gland and cavernous sinus. However, we removed parts of the diaphragma sellae with the superior surface of the tumor because the tumor was strongly adhered to it. The tumor was completely removed in a piecemeal fashion, and the pathology was compatible with renal cell carcinoma (Fig. 4). The post-operative course was uneventful and her vision recovered to its previous original state within one month after surgery. The clinical and radiological follow-up has been continued without adjuvant therapy and hormone replacement.
The pituitary gland is an uncommon site for the metastasis of systemic malignancies. Metastatic pituitary tumors constitute less than 1% of all surgical specimens from transsphenoidal surgery for sellar or parasellar tumors. Breast and lung cancer account for two thirds of pituitary metastases, although metastases from almost all cancers have been reported7,8). Additionally, renal cell carcinoma is a primary malignancy in only 2.6% of pituitary metastases5).
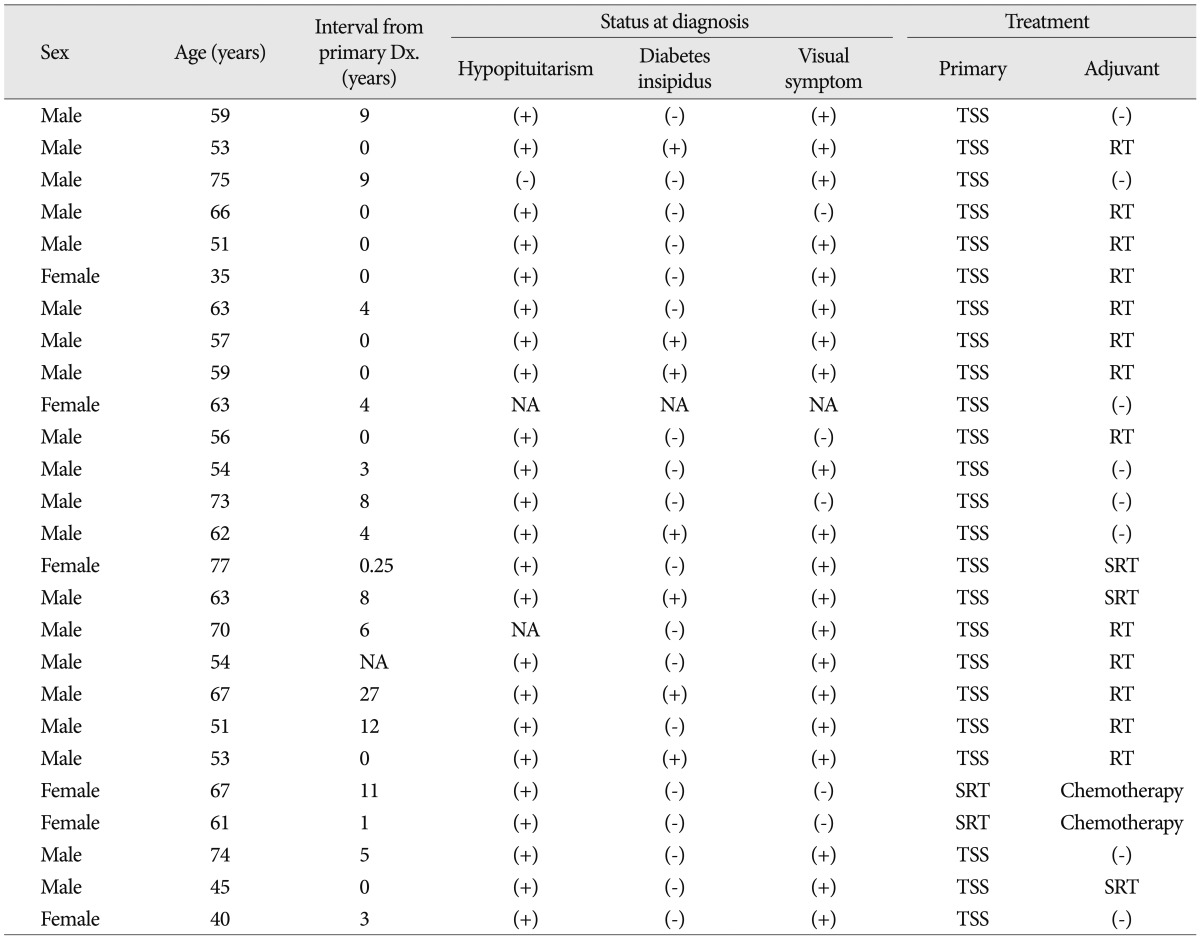
The differential diagnosis of pituitary metastases from other pathologies is challenging. Most metastatic pituitary tumors involve the posterior lobe6). A series of 190 symptomatic pituitary metastases identified the diabetes insipidus in 45.2% of patients4). The putative causes for frequent involvement of the posterior lobe were the relatively wide contact with the adjacent dura and the direct arterial supply from the hypophyseal arteries, contrasting with the anterior lobe from the hypophyseal portal system6). Therefore, the presence of diabetes insipidus or cavernous cranial neuropathy in patients with primary malignancy suggests to pituitary metastasis over relatively common pathologies such as adenoma and Rathke's cleft cyst. However, pituitary metastasis from renal cell carcinoma shows similar symptoms and signs to pituitary adenoma. A recent study has revealed that the anterior pituitary dysfunction is more prevalent (90%) and diabetes insipidus is less prevalent (24%) in patients with pituitary metastases from renal cell carcinoma compared to those with other metastatic tumors4). These clinical features make it difficult to differentiate the pituitary metastasis from renal cell carcinoma from common pituitary pathologies with clinical features except the rapid growth rate as the presented case. Additionally, visual field defects are more commonly found in patients with renal cell metastases (82%) than those with other primary malignancies. Therefore, the trans-sphenoid surgery was recommended as the first-line treatment in all cases in literatures and stereotactic radiotherapy was performed in patients refused the surgery1,2,4). A half of patients underwent the radiotherapy as the adjuvant treatment (Table 1).
In the reported case, we attempted to characterize a pituitary lesion with 18F-FDG PET at the time of presentation. However, the lesion did not show uptake in 18F-FDG PET, contrasting with the metastatic tumors in other sites, such as the adrenal gland and abdominal lymph nodes. The follow-up 18F-FDG PET, which was performed 2 months before the vision disturbance, also did not show increased uptake in the pituitary gland. Retrospectively, 18F-FDG PET was not helpful for differentiating the pituitary metastasis from other pathologies in the reported case. Recently, a report revealed that various pathological abnormalities were identified using MR imaging in 52.7% of patients who showed focal 18F-FDG accumulation3). Additionally, the most common entity was pituitary adenoma, followed in incidence by metastasis and lymphoma. However, there was no significant difference in standardized uptake values between malignant and benign lesions. Therefore, the specific diagnosis of a pituitary lesion with 18F-FDG is not yet possible.
There were some characteristic of pituitary metastasis on the MR imaging, such as high signal intensity on T2-weighted images with iso- or hypointense signals, loss of high signal intensity in the posterior lobe on T1-weighted images, and a rapidly growing sellar mass with infiltrating features. However, these findings cannot be the pathognomic criteria for the diagnosis of pituitary metastasis, with the exception of growth rate. The rapid development of the visual field defect and growth on the follow-up MR images provided the clues for diagnosis. Therefore, the follow-up interval should be shorter in patients with asymptomatic pituitary lesions and systemic malignancies.
References
1. Gopan T, Toms SA, Prayson RA, Suh JH, Hamrahian AH, Weil RJ. Symptomatic pituitary metastases from renal cell carcinoma. Pituitary. 2007; 10:251–259. PMID: 17541748.

2. Grossman R, Maimon S, Levite R, Ram Z. Multimodal treatment of hemorrhagic pituitary metastasis as first manifestation of renal cell carcinoma. World Neurosurg. 2013; 79:798.E1–798.E5. PMID: 22990000.

3. Hyun SH, Choi JY, Lee KH, Choe YS, Kim BT. Incidental focal 18F-FDG uptake in the pituitary gland : clinical significance and differential diagnostic criteria. J Nucl Med. 2011; 52:547–550. PMID: 21421711.

4. Kim YH, Lee BJ, Lee KJ, Cho JH. A case of pituitary metastasis from breast cancer that presented as left visual disturbance. J Korean Neurosurg Soc. 2012; 51:94–97. PMID: 22500201.

5. Komninos J, Vlassopoulou V, Protopapa D, Korfias S, Kontogeorgos G, Sakas DE, et al. Tumors metastatic to the pituitary gland : case report and literature review. J Clin Endocrinol Metab. 2004; 89:574–580. PMID: 14764764.

6. Kramer CK, Ferreira N, Silveiro SP, Gross JL, Dora JM, Azevedo MJ. Pituitary gland metastasis from renal cell carcinoma presented as a non-functioning macroadenoma. Arq Bras Endocrinol Metabol. 2010; 54:498–501. PMID: 20694412.

7. Max MB, Deck MD, Rottenberg DA. Pituitary metastasis : incidence in cancer patients and clinical differentiation from pituitary adenoma. Neurology. 1981; 31:998–1002. PMID: 7196526.

8. Sioutos P, Yen V, Arbit E. Pituitary gland metastases. Ann Surg Oncol. 1996; 3:94–99. PMID: 8770309.

9. Teears RJ, Silverman EM. Clinicopathologic review of 88 cases of carcinoma metastatic to the putuitary gland. Cancer. 1975; 36:216–220. PMID: 1203849.

Fig. 1
The T1-weighted coronal (A) and sagittal (B) magnetic resonance images used to evaluate the intermittent headache reveal a round, well-enhanced sellar mass. The pituitary stalk is slightly deviated to the left by the tumor, and no optic compression is noted. 18F-fluorodeoxyglucose positron emission tomography shows no increased uptake in the sellar mass (C) in contrast to the other metastatic lesions in the body (D).
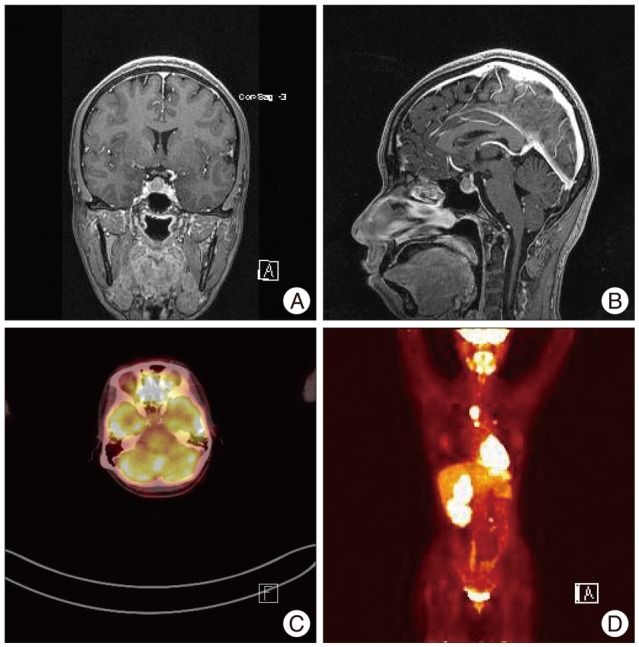
Fig. 2
The T1-weighted coronal (A) and sagittal (B) magnetic resonance images taken 6 months later show the markedly growing sellar mass compressing the optic chiasm.
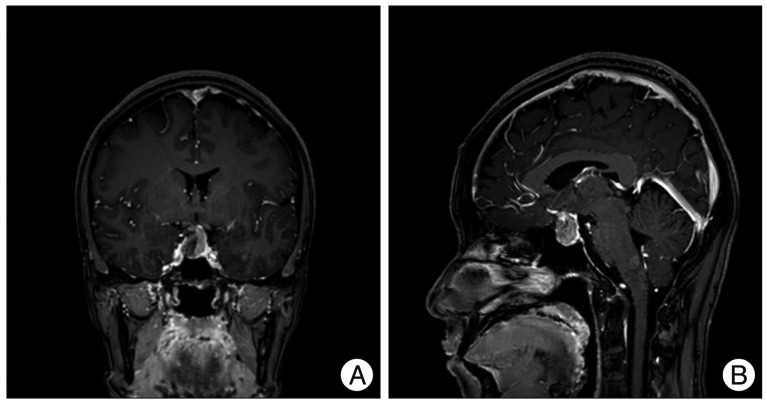
Fig. 3
The tumor was gray and moderately hard (A). It has a sharp margin between the pituitary gland (B) and the diaphragma sellae (C). The dissection plane between the tumor and the surrounding normal structures is not strongly adhesive.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download