This article has been
cited by other articles in ScienceCentral.
Abstract
Objective
We performed this study to investigate whether the use of closed-suction drainage following microvascular decompression (MVD) causes cerebrospinal fluid (CSF) leakage.
Methods
Between 2004 and 2011, a total of 157 patients with neurovascular compression were treated with MVD. MVD was performed for hemifacial spasm in 150 (95.5%) cases and for trigeminal neuralgia in 7 (4.5%) cases. The mean age of the patients was 49.8±9.6 years (range, 20-69). Dural substitutes were used in 44 (28.0%) patients. Ninety-two patients (58.6%) were underwent a 4-5 cm craniotomy using drainage (drainage group), and 65 (41.4%) did a small 2-2.5 cm retromastoid craniectomy without closed-suction drainage (no-drainage group).
Results
Eleven (7.0%) patients experienced CSF leakage following MVD based on the criteria of this study; all of these patients were in the drainage group. In the unadjusted analyses, the incidence of CSF leakage was significantly related with the use of closed-suction drainage following MVD (12.0% in the drainage group vs. 0% in the no-drainage group, respectively; p=0.003; Fisher's exact test). Those who received dural substitutes and the elderly (cut-off value=60 years) exhibited a tendency to develop CSF leakage (p=0.075 and p=0.090, respectively; Fisher's exact test). In the multivariate analysis, only the use of closed-suction drainage was significantly and independently associated with the development of CSF leakage following MVD (odds ratio=9.900; 95% confidence interval, 1.418 to infinity; p=0.017).
Conclusion
The use of closed-suction drainage following MVD appears to be related to the development of CSF leakage.
Go to :

Keywords: Microvascular decompression, Cerebrospinal fluid leakage, Closed-suction drainage, Hemifacial spasm, Trigeminal neuralgia
INTRODUCTION
Microvascular decompression (MVD) was introduced in the late 1960s and is a widely accepted treatment for neurovascular compression, including hemifacial spasm (HFS), trigeminal neuralgia (TN), and glossopharyngeal neuralgia. Technological advances, combined with microsurgical innovations, make this procedure effective and safe
9). Additionally, MVD offers significant advantages for both HFS and TN in terms of success rates without further treatment when compared with alternative treatments, such as botulinum toxin injections for HFS and radiofrequency lesioning of the gasserian ganglion for TN
5).
However, to obtain these benefits, patients are subjected to a slightly higher treatment risk, including the risk of cerebellar injury, hearing loss, cerebrospinal fluid (CSF) leakage, facial palsy, etc
9). Therefore, many efforts have been made to reduce the rates of complications following MVD via neurophysiologic monitoring and/or modified surgical techniques
2,
8-
10,
12). Currently, the rate of facial palsy following MVD is very low, and the rates of cerebellar injury and hearing loss have been down by half, approximately less than 1%
9). However, the rate of CSF leakage, often causing fatal meningitis, remains high and has been reported to range from approximately 1% to 5%
3,
9,
13,
14).
One of the most important aspects of MVD with respect to the avoidance of CSF leakage is a watertight dural closure
9). Moreover, the appropriate choice of dural substitutes can also lower the risk of CSF leakage
11,
12). Another important consideration may be the use of closed-suction drainage following MVD. Closed-suction drainage has traditionally been used to prevent postoperative hematoma or in cases of uncertain hemostasis
6). However, the quoted incidence of intracranial hematoma following MVD is only 0.4-0.5%
5,
7). Moreover, epidural hematoma at the surgical site, for which only closed-suction drainage can work, appears to be even rarer. Furthermore, closed-suction drainage following MVD can cause CSF leakage (Jacques Magnan; personal communication, September 9, 2010). However, no studies have been performed to address this issue.
We compared a traditional retrosigmoid suboccipital craniotomy using closed-suction drainage to a minimally invasive small craniectomy without drainage. We performed this study to investigate whether closed-suction drainage following MVD causes CSF leakage by comparing the incidences of CSF leakage following MVD for both of these surgical procedures.
Go to :

MATERIALS AND METHODS
Between 2004 and 2011, a total of 157 patients with neurovascular compression were treated with MVD. Of these, 106 (67.5%) patients were female. MVD was performed for HFS in 150 (95.5%) cases and for TN in 7 (4.5%) cases. The mean age was 49.8±9.6 years (range, 20-69). Our surgical procedures for MVD included a traditional retromastoid craniotomy and a minimally invasive approach in which a small 2-2.5 cm retromastoid craniectomy using a bony landmark of the incisura mastoidea or the mastoid notch, as described elsewhere
4,
9). The surgical procedure was decided as each surgeon's preference.
A traditional retromastoid craniotomy with closed-suction drainage
A three-point head-fixation was applied while the patient is in the supine lateral position. After shaving the area 4 to 5 cm behind the ear, the mastoid tip and notch were identified and marked. A lazy-S skin incision of 8 to 10 cm in length and a half above the mastoid notch was made along the hair line. A keyhole was drawn on the asterion, and the junction of the transverse sinus and sigmoid sinus was identified. A bone flap of approximately 4 cm in size was removed using a high-speed drill, and bony exposure was laterally extended to identify the posterior margin of the sigmoid sinus. The incision of the dura mater was made along the posterior margin of the sigmoid sinus. Following decompression of the offending vessel(s) from the cranial nerves, the bone edges of the mastoid air cells were thoroughly sealed with bone wax. A watertight dural closure was subsequently performed, and dural substitutes were used as necessary. Fibrin glue was applied on the epidural space, and the bone flap was repositioned usually with a titanium mesh. Before approximation of the deep and superficial muscles, a closed-suction drainage system (Hemovac®; generally 400 cc) was inserted into the subgaleal space between the bone flap and the muscle layer. Early mobilization of the patients was encouraged on postoperative day 1, and the patients were discharged following resolution of postoperative dizziness and headache.
A mastoid notch keyhole craniectomy without closed-suction drainage
With the patient in the supine position, the head was rotated approximately 20 to 30° away from the affected side without the use of head-fixation. The mastoid tip and notch were identified and marked, and a 1 cm-wide area was shaved behind the hairline. A 4 to 5 cm curvilinear skin incision is made along the hairline, in which three quarters were below the mastoid notch in cases of HFS and a half above the mastoid notch in cases of TN. Following the identification of the digastric groove, a 2 to 2.5 cm craniectomy was performed below the digastric groove for HFS and above the digastric groove for TN
4,
9). An incision of the dura mater was made along the inferoposterior margin of the sigmoid sinus. The offending vessel(s) were decompressed from the cranial nerves, and the bone edges of the mastoid air cells were waxed, as described above. A watertight dural closure was subsequently performed. If required, pieces of muscle from the adjacent neck muscles were used to perform the plugging muscle method, as described elsewhere
12). Following application of fibrinogen/thrombin-based collagen fleece (TachoComb®; Nycomed, Linz, Austria) for additional sealing of the dural incision and to achieve hemostasis of the outer surface of the exposed dura mater, a cranioplasty was performed usually using polymethylmethacrylate bone cement. Finally, the deep and superficial muscles were approximated without using closed-suction drainage. The remaining procedures and postoperative care were the same as described above.
Definition of CSF leakage following MVD and statistical analysis
CSF leakage following MVD was defined as follows : 1) rhinorrhea, otorrhea, pseudomeningocele, and/or an incisional leak at any time following MVD irrespective of the development of central nervous system infections
1); 2) middle ear effusions, generally accompanied by ear fullness, identified by thorough otological evaluations
7); 3) CSF leakage developing on postoperative day 2 or later, considering the time required for drainage of the fluid that potentially flowed into the mastoid air cells before waxing of the inlet during the procedure; and 4) persistence of CSF leakage for 2 or more days; in cases of middle ear effusion, effusion was identified at the follow-up otological evaluation 2 or 3 days following the initial exam.
The duration of closed-suction drainage was defined as the interval between skin closure to the removal or clamping of the drainage system. A single rater, who was blind to the clinical outcome data and the development of CSF leakage, reviewed all of the immediate postoperative computed tomography (CT) scans. This was performed to determine whether the mastoid air cells were opened during MVD or not, which may influence the development of middle ear effusion due to CSF leakage. The CT scan was obtained at a 2.5-5-mm thickness (Brilliance CT; Philips Medical Systems, WA, USA).
The clinical outcome of the neurovascular compression with respect to the surgical method was not a principal concern of this study and was not evaluated. All of the patient data were based on information that was contained in hospital charts. The radiological studies were collected in accordance with the case record form, which was approved by the institutional review board.
The incidence of CSF leakage was compared between the two groups of patients using Fisher's exact test. Student's t-test was performed to compare the means of the continuous variables between the groups. To reduce the probability of type II errors considering the modest sample size, variables were considered for the multivariate analysis only if they were associated with a dependent variable in each analysis at the p<0.10 level. A multiple logistic regression analysis was used to adjust the variables. When the estimate of the coefficient was zero or extreme, exact logistic regression was used to obtain a median unbiased estimate. A p value of 0.05 was considered significant. These statistical analyses were performed using PASW Statistics® software version 17.0.2 (SPSS Inc., Chicago, IL, USA) and the STATA software package version 12.0 (Stata Corp., College Station, TX, USA).
Go to :

RESULTS
Ninety-two (58.6%) patients underwent MVD using closed-suction drainage and were entered into the 'drainage group'. MVD without closed-suction drainage was performed in 65 (41.4%) patients, designated the 'no-drainage group'. In 44 (28.0%) patients, dural substitutes, such as Neuro-Patch® (B. Braun) or muscle pieces were used; a primary watertight dural closure without the use of dural substitutes was performed in the remaining patients. The mastoid air cells were opened during the surgery in 78 (49.7%) patients based on the routine postoperative CT scan.
Among 92 patients in the drainage group, the closed-suction drainage was removed or clamped due to CSF drainage via the drainage system in 57 patients (62.0%), due to air drainage in 10 patients (10.9%), and due to no or very little drainage in 23 patients (25.0%). In two (2.2%) patients, the closed-suction drainage was removed due to an additional surgery for postoperative supratentorial epidural hematoma. The mean duration of the closed-suction drainage was 20.9±11.6 hours (range, 0-48.3). In two (2.2%) patients, drainage was not performed following the placement of the closed-suction drainage because the CSF was drained immediately after the surgery; the drainage was therefore subsequently clamped and removed. The clinical and surgical characteristics of the patients are summarized in
Table 1.
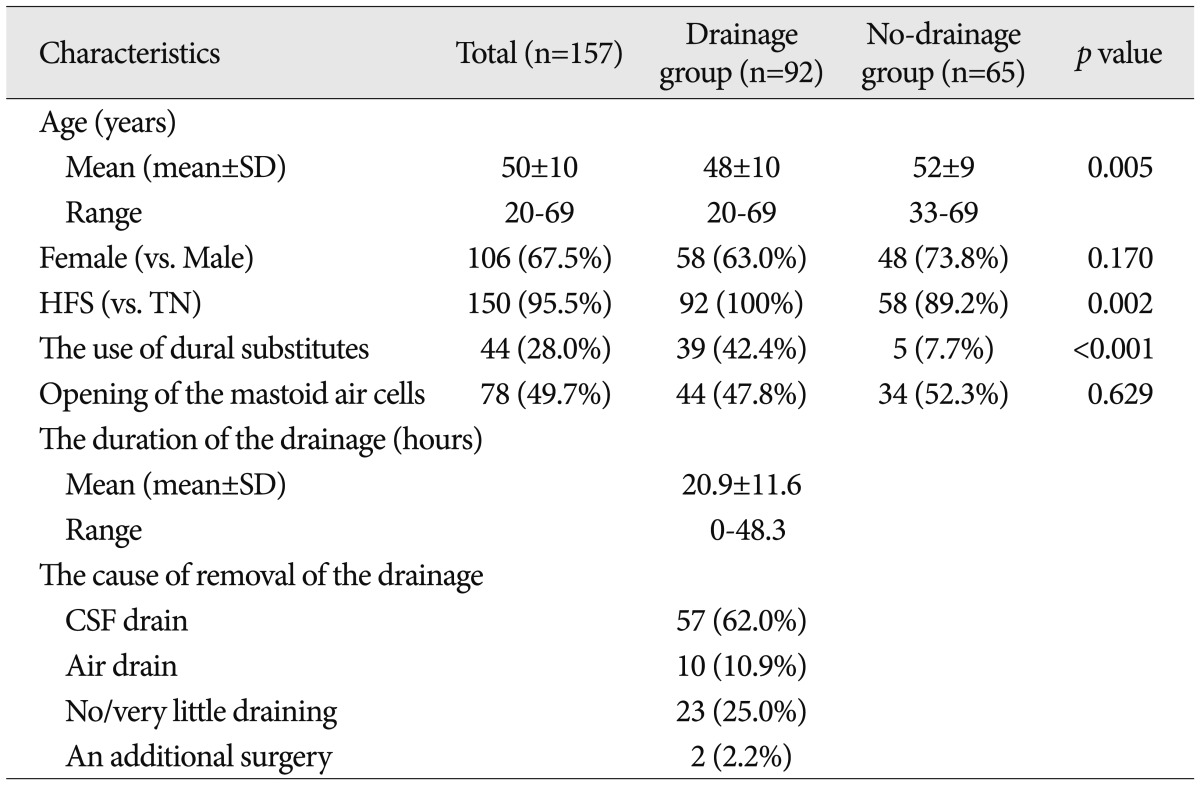
Table 1
The clinical characteristics of the patients

CSF leakage and closed-suction drainage
A total of 11 (7.0%) patients experienced CSF leakage following MVD based on the criteria of this study. Interestingly, all of these patients were in the drainage group (12.0% of the drainage group). The details of the observed CSF leakage are summarized in
Table 2. Eight of these patients were conservatively treated with bed rest and local management, such as a mastoid compression dressing. In two of these patients, continuous lumbar drainage was put in place for 5 days, and CSF leakage was successfully controlled. One 50-year-old male, whose drainage system drained air immediately following the surgery, underwent an additional surgery on the 13th postoperative day for a cerebellar abscess combined with meningitis and middle ear effusion (
Fig. 1).
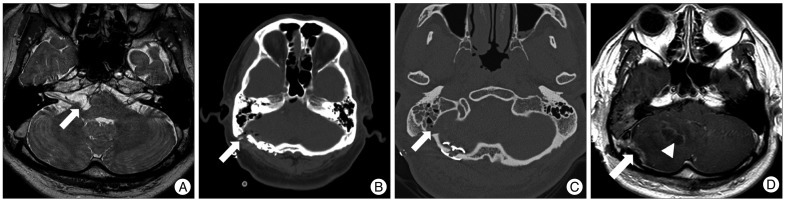 | Fig. 1A 50-year-old male, whose drainage system drained air immediately following MVD, underwent an additional surgery on the 13th postoperative day for a cerebellar abscess combined with meningitis and middle ear effusion. A : A loop of the posterior inferior cerebellar artery (arrow) offending the root exit zone of the right facial nerve in the supra-olivary fossette. B : The immediate postoperative computed tomography scan shows the site of the surgery and a portion of the closed-suction drainage (arrow). C : A follow-up evaluation using temporal bone computed tomography for middle ear effusion and fever shows an opened mastoid air cell (arrow) and air density near the wound on the second postoperative day. Fluid in the mastoid air cells can also be identified. D : Follow-up magnetic resonance imaging on the sixth postoperative day shows abnormal lesions with contrast enhancement near the bone flap (arrow) and in the middle of the right cerebellar hemisphere (arrowhead). The mass effect is caused by the cerebellar abscess slightly displacing the pons to the left. 
|
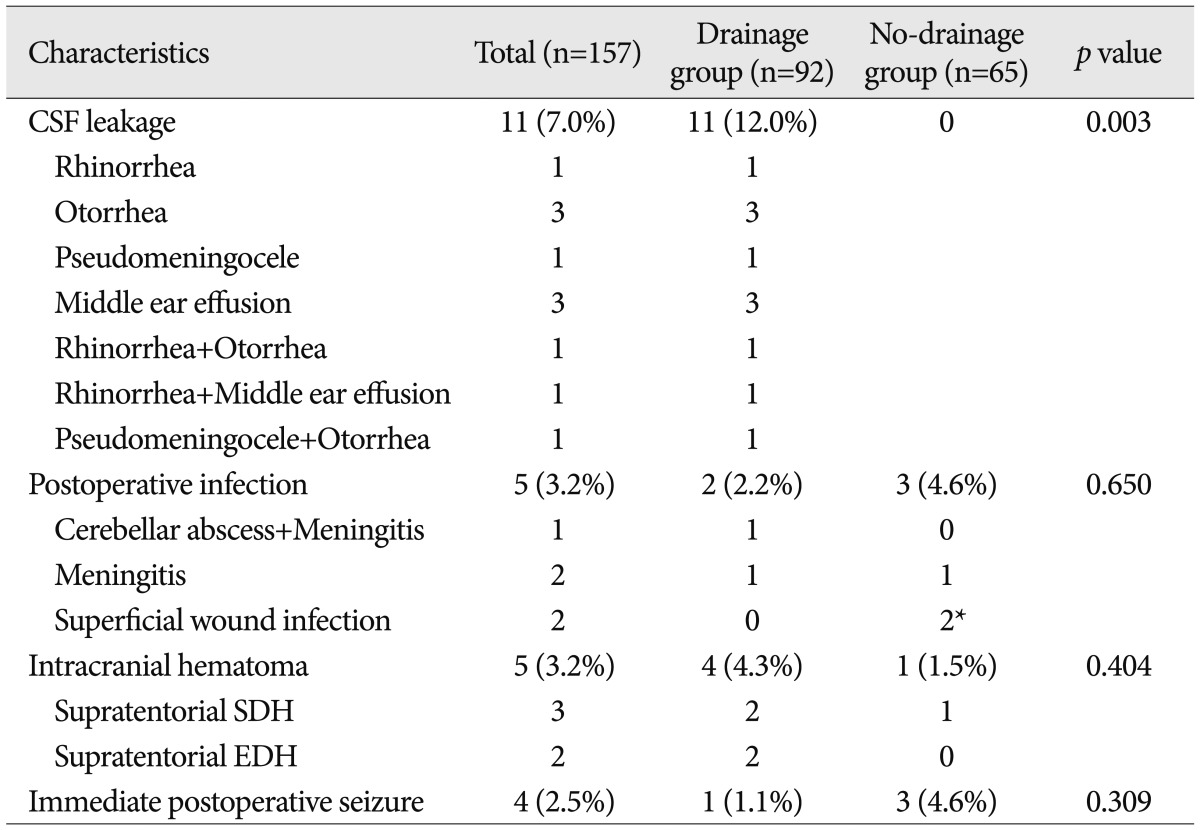
Table 2
CSF leakage and other complications following MVD

In the no-drainage group, one patient exhibited a trace of slightly yellow fluid on her pillow on postoperative day 4. However, no evidence of middle ear effusion or wound leakage was identified, even following a thorough otological evaluation. No further event suggesting CSF leakage developed thereafter. Thus, based on our criteria of CSF leakage, no patient in the no-drainage group experienced CSF leakage following MVD.
In the unadjusted analyses, the incidence of CSF leakage was significantly related with the use of closed-suction drainage following MVD (12.0% in the drainage group vs. 0% in the no-drainage group, respectively; p=0.003; Fisher's exact test). The use of dural substitutes tended to increase the risk of CSF leakage. Six (13.6%) of the 44 patients for whom dural substitutes were used experienced CSF leakage, as did 5 (4.4%) of the 113 patients who did not receive dural substitutes; however, this effect did not reach statistical significance (p=0.075; Fisher's exact test). Lastly, the elderly patients (cut-off value=60 years) also exhibited a trend toward an increased risk of CSF leakage (p=0.090; Fisher's exact test); however, this effect also did not reach statistical significance. The opening of the mastoid air cells during surgery was unrelated to the development of CSF leakage following MVD (p=0.369; Fisher's exact test).
In the multivariate analysis, only the use of closed-suction drainage was significantly and independently related with the development of CSF leakage following MVD (odds ratio=9.900; 95% confidence interval, 1.418 to infinity;
p=0.017). The results of the statistical analyses are summarized in
Table 3.
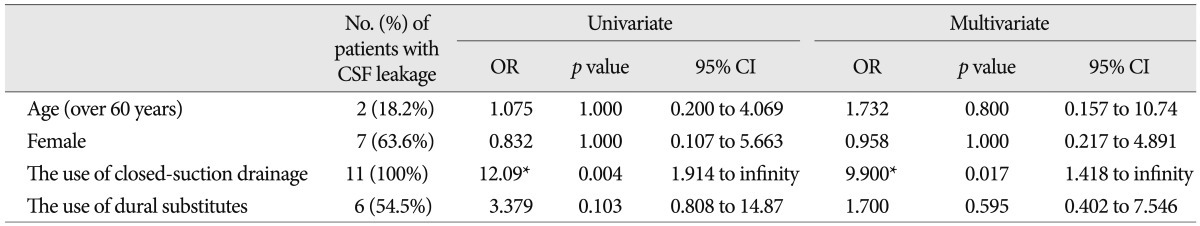
Table 3
The results of the multiple logistic regression analyses of the risk factors for developing CSF leakage

Other complications
A postoperative infection developed in 5 (3.2%) patients. One patient in the drainage group experienced a cerebellar abscess following a middle ear effusion, as mentioned above. Another patient in the drainage group developed pseudomeningocele combined with meningitis and was treated conservatively. In the no-drainage group, 3 patients experienced postoperative infection; one with aseptic meningitis and two successive cases of superficial wound infection caused by Serratia marcescens. These latter infections developed during an outbreak in the operating room.
An asymptomatic small amount of supratentorial subdural hematoma was observed on the routine immediate postoperative CT scan in 3 (1.9%) patients (2 in the drainage group and 1 in the no-drainage group). Remote supratentorial epidural hematoma developed in 2 (1.3%) patients. Interestingly, both of these patients were in the drainage group. These patients underwent an additional surgery and recovered without any neurological deficits.
Two (1.3%) patients in the no-drainage group developed ecchymosis in the neck near the surgical wound; however, they did not exhibit related symptoms or require further management. Interestingly, 4 (2.5%) patients experienced an immediate postoperative seizure without any evidence of intracranial hemorrhage. These patients also recovered without any neurological deficits. There was no mortality in either group following MVD.
Go to :

DISCUSSION
The role of closed-suction drainage following surgery has been thoroughly investigated for various procedures to prevent postoperative hematoma and infection-related hematoma in the dead space
6). However, the usefulness of closed-suction drainage following MVD has not been thoroughly investigated despite the quoted low incidence of intracranial hematoma following MVD and the potential harm of closed-suction drainage. Fortunately, we were able to address this issue by virtue of our modification of the MVD procedure, which improves the surgical outcome and reduces the rates of complications. Based on our data, closed-suction drainage appears to be significantly associated with CSF leakage and postoperative infection following MVD. And the majority of the placed drainage systems were removed or clamped within one day or immediately after the surgery. Furthermore, considering the rate of 10% (one patient) of cerebellar abscess and meningitis among the 10 patients who exhibited air drainage via their closed-suction drainage systems, air drainage may cause a fatal central nervous system infection, such as a cerebellar abscess.
Although the pathogenesis of CSF leakage following MVD is still debated, one of the most important aspects of MVD with respect to the avoidance of CSF leakage is a primary watertight dural closure
9,
12). Many dural substitutes have been developed and are used to make a watertight dural closure when a primary watertight dural closure is impossible due to shrinkage of the dural margins. The appropriate choice of dural substitute also appears to affect the risk of CSF leakage
11). Nonetheless, the use of dural substitutes tended to encourage CSF leakage in our study, although this effect did not reach statistical significance and was dropped from the multivariate analysis.
One of the most notable aspects of this study was the definition of CSF leakage. Many studies have examined CSF leakage following lateral skull base surgeries for neurovascular compression, vestibular schwannoma, etc
1,
7,
15). However, these studies did not precisely define CSF leakage. In our study, middle ear effusion was regarded as CSF leakage when it was identified by otological evaluations and persisted for 2 or more days following MVD. This definition appears to be a possible reason for the relatively high incidence of CSF leakage (12.0%) observed in the drainage group. However, considering that not even middle ear effusion developed in the no-drainage group, for which there was a higher incidence of opening of the mastoid aircells, closed-suction drainage appears to be one of major causes of CSF leakage following MVD.
Another interesting finding was that remote supratentorial epidural hematoma developed only in the drainage group. Although the pathogenesis of remote supratentorial epidural hematoma following MVD is unclear, excessive CSF draining via the closed-suction drainage system may be a principal factor. The same explanation may be applied for supratentorial subdural hematoma, although this condition did not cause any neurological symptoms in our cohort.
This study has several limitations, including a retrospective design, a relatively small sample size, and possible biases, such as the use of dural substitutes and different surgical methods. However, the effects of these limitations on the primary conclusions of this manuscript are likely minimal.
Go to :

CONCLUSION
The use of closed-suction drainage following MVD appears to be related to the development of CSF leakage.
Go to :

Acknowledgements
This study was supported by grant no. 02-2011-006 from the SNUBH Research fund.
Go to :

References
1. Allen KP, Isaacson B, Purcell P, Kutz JW Jr, Roland PS. Lumbar subarachnoid drainage in cerebrospinal fluid leaks after lateral skull base surgery. Otol Neurotol. 2011; 32:1522–1524. PMID:
21956598.

2. Bien AG, Bowdino B, Moore G, Leibrock L. Utilization of preoperative cerebrospinal fluid drain in skull base surgery. Skull Base. 2007; 17:133–139. PMID:
17768443.

3. Broggi G, Ferroli P, Franzini A, Servello D, Dones I. Microvascular decompression for trigeminal neuralgia : comments on a series of 250 cases, including 10 patients with multiple sclerosis. J Neurol Neurosurg Psychiatry. 2000; 68:59–64. PMID:
10601403.

4. Hitotsumatsu T, Matsushima T, Inoue T. Microvascular decompression for treatment of trigeminal neuralgia, hemifacial spasm, and glossopharyngeal neuralgia : three surgical approach variations : technical note. Neurosurgery. 2003; 53:1436–1441. discussion 1442-1443. PMID:
14633313.
5. Kalkanis SN, Eskandar EN, Carter BS, Barker FG 2nd. Microvascular decompression surgery in the United States, 1996 to 2000 : mortality rates, morbidity rates, and the effects of hospital and surgeon volumes. Neurosurgery. 2003; 52:1251–1261. discussion 1261-1262. PMID:
12762870.
6. Kanayama M, Oha F, Togawa D, Shigenobu K, Hashimoto T. Is closed-suction drainage necessary for single-level lumbar decompression? : review of 560 cases. Clin Orthop Relat Res. 2010; 468:2690–2694. PMID:
20091386.

7. Li D, Wang H, Fan Z, Fan Z. Complications in retrosigmoid cranial nerve surgery. Acta Otolaryngol. 2010; 130:247–252. PMID:
19593681.

8. Li N, Zhao WG, Pu CH, Shen JK. Clinical application of artificial dura mater to avoid cerebrospinal fluid leaks after microvascular decompression surgery. Minim Invasive Neurosurg. 2005; 48:369–372. PMID:
16432788.

9. McLaughlin MR, Jannetta PJ, Clyde BL, Subach BR, Comey CH, Resnick DK. Microvascular decompression of cranial nerves : lessons learned after 4400 operations. J Neurosurg. 1999; 90:1–8. PMID:
10413149.

10. Miyazaki H, Deveze A, Magnan J. Neuro-otologic surgery through minimally invasive retrosigmoid approach : endoscope assisted microvascular decompression, vestibular neurotomy, and tumor removal. Laryngoscope. 2005; 115:1612–1617. PMID:
16148704.

11. Moskowitz SI, Liu J, Krishnaney AA. Postoperative complications associated with dural substitutes in suboccipital craniotomies. Neurosurgery. 2009; 64(3 Suppl):ons28–ons33. discussion ons33-ons34. PMID:
19240570.

12. Park JS, Kong DS, Lee JA, Park K. Intraoperative management to prevent cerebrospinal fluid leakage after microvascular decompression : dural closure with a "plugging muscle" method. Neurosurg Rev. 2007; 30:139–142. discussion 142. PMID:
17221266.

13. Patel A, Kassam A, Horowitz M, Chang YF. Microvascular decompression in the management of glossopharyngeal neuralgia : analysis of 217 cases. Neurosurgery. 2002; 50:705–710. discussion 710-711. PMID:
11904019.
14. Samii M, Günther T, Iaconetta G, Muehling M, Vorkapic P, Samii A. Microvascular decompression to treat hemifacial spasm : long-term results for a consecutive series of 143 patients. Neurosurgery. 2002; 50:712–718. discussion 718-719. PMID:
11904020.
15. Selesnick SH, Liu JC, Jen A, Carew JF. Management options for cerebrospinal fluid leak after vestibular schwannoma surgery and introduction of an innovative treatment. Otol Neurotol. 2004; 25:580–586. PMID:
15241238.

Go to :






 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download