Abstract
The abducens nerve paresis generally can aid in the presumptive diagnosis of abducens schwannoma along with the typical radiological features of schwannomas. The authors present a case of a 76-year-old male patient with a abducens schwannoma without abducens nerve paresis. Peroperatively, abducens nerve located in the cerebellopontine cistern had normal in contour and diameter, despite the mass originated from this nerve. We hypothesize that anatomic location of abducens nerve may affect the vector of tumor growth to prevent destruction of its origin, the abducens nerve.
Intracranial schwannomas, one of the most frequent primary brain tumors, commonly arise from the glial-schwann myelination junction of the sensory nerve, such as the vestibular and trigeminal cranial nerves (CNs). Although they usually develop within motor nerve fibers, especially in association with neurofibromatosis, sporadic involvement has also been described in the facial, hypoglossal, abducens and trochlear nerves3). Till date, only 20 cases of abducens nerve schwannomas have been reported, including the present case2,6,7). Along with the typical radiological features of schwannomas, abducens palsy has been regarded as a preoperative diagnostic clue. However, in rare cases including this one5,7), the absence of the abducens nerve sign raised difficulties in clinical presumption before surgery. According to the location of the tumor, abducens schwannomas can be classified into cisternal, cavernous, and cisternocavernous groups1). Interestingly, three cases without abducens palsy were apparently categorized into a cisternal type. All the tumors that involved the cavernous sinus showed abducens palsy, preoperatively. Although the absence of trochlear paresis, even in large trochlear schwannomas, can be explained by the long length of cavernous segment of trochlear nerve1), little is known about similar phenomenon in abducens schwannoma. Based on the surgical experience of abducens schwannoma without the origin nerve deficit, the authors discuss a possible mechanism to explain this rare phenomenon according to the anatomical relation between the tumor and surrounding arachnoid structures.
A 76-year-old male patient, without significant medical illnesses, presented with right facial numbness and hearing impairment for a month. The patient showed no neurological symptoms or signs except hypoestheia/hypoalgesia of the trigeminal nerve and mild hearing impairment on the right side. Magnetic resonance imaging revealed a multi-septated cystic mass on the right cerebellopontine angle (Fig. 1A, B). The epidermoid cyst was excluded as a possible preoperative diagnosis based on diffusion-weighted image (Fig. 1C). There was no enlargement of the internal auditory canal on computed tomography scans with bone window (Fig. 1D). Pure tone audiometry and speech discrimination score showed almost normal hearing status. There were no limitations observed with the extraocculomotor movement (EOM). The preoperative diagnosis was a trigeminal schwannoma which was mainly cystic nature. Surgical resection was performed with the standard right lateral suboccipital approach. A thin-walled cystic mass displaced the lower CNs inferolaterally, the vestibulo-facial CN complex laterally, and the trigeminal nerve superiorly. After being fenestrated through its cyst wall, the mass was found to be originating from the abducens nerve. The abducens nerve seemed normal in contour and diameter, although it compressed other surrounding CNs. The tumor and its origin CN were medially located anterior to the pontine membrane and coursed within the cerebellopontine cistern (Fig. 2). Following a meticulous dissection, the cystic tumor was totally removed without any injury of the origin CN. Pathological examination revealed a schwannoma (Fig. 3). Postoperatively, the patient complained of transient diplopia without definitive EOM limitation. His symptom disappeared by 3 months after operation.
Considering the fact that the abducens palsy, with/without neurological signs from other CN involvement or brain stem compression, leads to a preoperative diagnosis of abducens schwannoma, the absence of origin CN deficits can make it difficult to presume a tentative diagnosis and select the appropriate treatment strategy. It is difficult to explain the inconsistency between tumor involvement of a cranial nerve and clinical non-manifestation. In Nakamura et al.'s5) case, the tumor was located in the prepontine area and demonstrated a large-size and solid nature. They postulated that the possible mechanism for the absence of clinical manifestation may be due to neuronal tolerance induced by extremely slow growth of tumor. Another case, reported by Vachata and Sames7), demonstrated that the small and cystic nature of the tumor could be associated with less compression against its origin CN and the absence of preoperative abducens paresis.
In our present case, interesting peroperative findings were found to provide us some clues to understand the atypical clinical features. First, the abducens nerve was maintained in normal contour and diameter despite being severely adhered by tumor. The trigeminal nerve and vestibule-facial nerve complex, on the other hand, were displaced and appeared somewhat concaved against the tumor. These features could be correlated with preoperative symptoms, facial numbness and minimal hearing impairment. Second, there was an arachnoid membrane covering the tumor and its origin, the abducens nerve. This arachnoid membrane, demarcating brainstem from tumor and its origin nerve, was considered as the anterior pontine membrane. The anterior pontine membrane is a bounding line between the prepontine cistern and cerebellopontine cistern. Generally, the trigeminal nerve and vestibule-facial nerve complex are contained in the cerebellopontine cistern, while the abducens CN in the prepontine cistern8). However, a recent detailed study for intracranial cistern revealed that the abducens nerve ascends just lateral to the anterior pontine membrane within the cerebellopontine cistern4).
Taken together with above mentioned facts, some possible mechanisms can be postulated to explain the absence of abducens nerve deficit. In this case, the tumor had originated from the abducens nerve in the cerebellopontine cistern. Because the anterior pontine membrane was located medially to abducens nerve and played a possible role in anatomical barrier, the vector of tumor growth shifted to lateral aspect of the cerebellopontine cistern. This growth pattern led to a more compression effect on the nerve structures in the cerebellopontine cistern, such as the trigeminal nerve and the vestibulo-facial nerve complex, than on its origin abducens nerve, and consequently to displaced and concaved changes in compressed CNs, in contrary to the preserved abducens nerve.
Although it is difficult to conclude the exact mechanisms for the lack of origin CN palsy in abducens schwannoma, the anatomical location of the abducens nerve may affect the direction of growth of the main tumor and the severity of compression of the surrounding CNs. If the abducens nerve is located in the cerebellopontine cistern, the anterior pontine membrane, just medial to abducens nerve, may cause the shift in the tumor growth vector to the lateral aspect and result in compression of the surrounding CNs, instead of affecting the origin, abducens nerve.
References
1. Celli P, Ferrante L, Acqui M, Mastronardi L, Fortuna A, Palma L. Neurinoma of the third, fourth, and sixth cranial nerves : a survey and report of a new fourth nerve case. Surg Neurol. 1992; 38:216–224. PMID: 1440207.

2. Erlich SA, Tymianski M, Kiehl TR. Cellular schwannoma of the abducens nerve : case report and review of the literature. Clin Neurol Neurosurg. 2009; 111:467–471. PMID: 19200646.

3. Lantos PL, Vandenberg SR, Kleihues P. Tumours of the Peripheral Nerve. In : Graham DI, Lantos PL, editors. Greenfield's neuropathology. London, UK: Arnold;2001. p. 713–717.
4. Matsuno H, Rhoton AL Jr, Peace D. Microsurgical anatomy of the posterior fossa cisterns. Neurosurgery. 1988; 23:58–80. PMID: 3173665.

5. Nakamura M, Carvalho GA, Samii M. Abducens nerve schwannoma : a case report and review of the literature. Surg Neurol. 2002; 57:183–188. discussion 188-189. PMID: 12009546.
6. Park JH, Cho YH, Kim JH, Lee JK, Kim CJ. Abducens nerve schwannoma : case report and review of the literature. Neurosurg Rev. 2009; 32:375–378. discussion 378. PMID: 19418078.

7. Vachata P, Sames M. Abducens nerve schwannoma mimicking intrinsic brainstem tumor. Acta Neurochir (Wien). 2009; 151:1281–1287. PMID: 19357806.

8. Yasargil MG, Kasdaglis K, Jain KK, Weber HP. Anatomical observations of the subarachnoid cisterns of the brain during surgery. J Neurosurg. 1976; 44:298–302. PMID: 1082498.

Fig. 1
A and B : Preoperative axial T2-weighted and Gd-enahnced MRI demonstrating a large sized cystic mass on the right cerebellopontine angle with peripheral rim enhancement and internal multi-septations. C : Diffusion-weighted image showing no diffusion restriction in the cystic fluid. D : Preoperative CT scan with bone window change revealing no enlargement of internal auditory canal.
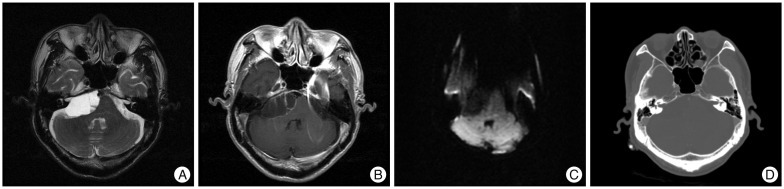
Fig. 2
A : Peroperative photograph before fenestration of cyst wall showing thin walled cyst mass laterally displacing the vestibulo-facial nerve complex. B : Peroperative photograph revealing anatomical relationships among the anterior pontine membrane, the mass, and origin abducens nerve. Note that the anterior pontine membrane medially located from the mass and its origin nerve and enveloped them. It became a demarcation landmark between the prepontine cistern and cerebellopontine cistern. C : Peroperative photograph after resection of the mass showing the origin abducens nerve with normal contour and diameter, and upward-displaced trigerminal nerve with partly concaved against the previous mass. T : tumor, VI : abducens nerve, V : trigeminal nerve, BS : brainstem, APM : anterior pontine membrane, VII-VIII : vestibulo-facial nerve complex.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download