Abstract
Objective
Several scales are currently used to assess occlusion rates of coiled cerebral aneurysms. This study compared these scales as predictors of recanalization.
Methods
Clinical data of 827 patients harboring 901 aneurysms treated by coiling were retrospectively reviewed. Occlusion rates were assessed using angiographic grading scale (AGS), two-dimensional percent occlusion (2DPO), and volumetric packing density (vPD). Every scale had 3 categories. Followed patients were dichotomized into either presence or absence of recanalization. Kaplan-Meier analysis was conducted, and Cox proportional hazards analysis was performed to identify surviving probabilities of recanalization. Lastly, the predictive accuracies of three different scales were measured via Harrell's C index.
Results
The cumulative risk of recanalization was 7% at 12-month, 10% at 24-month, and 13% at 36-month of postembolization, and significantly higher for the second and third categories of every scale (p<0.001). Multivariate-adjusted hazard ratios (HRs) of the second and third categories as compared with the first category of AGS (HR : 3.95 and 4.15, p=0.004 and 0.001) and 2DPO (HR : 4.87 and 3.12, p<0.001 and 0.01) were similar. For vPD, there was no association between occlusion rates and recanalization. The validated and optimism-adjusted C-indices were 0.50 [confidence (CI) : -1.09-2.09], 0.47 (CI : -1.10-2.09) and 0.44 (CI : -1.10-2.08) for AGS, 2DPO, and vPD, respectively.
Coiling is being increasingly used as an alternative to surgical clipping for cerebral aneurysms. Despite substantial advances in both endovascular instrumentations and techniques, however, there are still concerns about its efficacy and durability. Recanalization and retreatment after coiling are known to occur in 10-40%1,6,12,13,15,16) and 5-10% of follow-up cases, respectively2,16,18-20). Coil manufacturers have made efforts to develop new coils to decrease the rates of recanalization. These include bioactive coils using polyglycolic or combined polyglycolic/polylactic acid (PGLA), nylon/Dacron/PGLA fibers, and hydrogel, which were designed to promote healing of aneurysms. The strategy of increasing packing rates has also been implemented to reduce the recanalization rate, because lower packing was found to be strongly associated with recanalization. In addition, there is no evidence that attempting more complete aneurysm occlusion is associated with greater procedure-related complication risks5). However, different institutions reported the occlusion rates using different assessment scales, and the occlusion rates were not assessed directly by one or two observers but abstracted from procedural reports submitted by various practitioners in prospective multicenter studies5,12,16,19,24). It may have led to unreliable results due to unstandardized measurements.
The assessment scales include angiographic grading scale (AGS) divided into 2, 3, or 4 categories and a percentage scale, classified as two-dimensional percent occlusion (2DPO), such as 100%, 90-99%, and <90% occlusion, and calculated volumetric packing density (vPD). Of these scales, vPD is the only objective scale without regard to validity, because AGS and 2DPO are usually determined by visual estimation. The association between vPD and recanalization of coiled aneurysms has been reported previously6,7,21,22).
Assessment scales have to be objective and standardized if recanalization after coiling is to be predicted reliably by the initial occlusion rates. However, visually estimated AGS and 2DPO, although both are widely used with ease, are apparently subjective. Calculated 2DPO and vPD also are not free from errors. The best scale as a predictor of recanalization is yet to be established. The aim of the present study was to determine whether representative scales in current use were appropriate to give accurate information on the risk of recanalization and which scale was the best statistically and practically.
This retrospective study was approved by the institutional review board at our institution and informed consent was waived.
Between April 2003 and April 2010, 946 patients harboring 1056 aneurysms underwent initial treatment with detachable coils at our institution. Three hundred and twenty six (n=326) aneurysms were excluded with the following reasons : unavailable 3D data (n=45), follow-up loss (n=48), postsurgical coiling (n=15), intentional multi-staged procedure (n=3), dissecting aneurysms (n=5), and the use of bioactive coils, such as HydroCoil™ (n=39) and PGLA-coated coil (n=171).
Of the remaining 671 patients harboring 730 aneurysms, 494 (67.7%) patients were female and 236 (32.3%) were male. Their age ranged from 23 to 84 years, with a mean age of 56.46 years. Five hundred and forty eight aneurysms (75.1%) were unruptured and 182 (24.9%) were ruptured. Three hundred and eighty two aneurysms (52.3%) were lobulated with two or more sacs, and 348 (47.7%) were single saccular. Maximal sizes of aneurysms ranged from 2.0 to 29.0 mm, with a mean size of 5.86 mm. Aneurysm locations were as follows : anterior cerebral artery in 198 (27.1%), middle cerebral artery in 83 (11.4%), internal cerebral artery in 366 (50.1%), and posterior circulation in 83 (11.4%). Endovascular procedures were performed following a standardized protocol8). Aneurysms were packed as densely as possible with only bare platinum coils.
Three different scales were used to assess the occlusion rates : AGS, 2DPO, and vPD. For AGS, 3 categories were defined, including complete occlusion, residual neck, and residual aneurysm17). For 2DPO, 3 categories were defined; 100%, 90-99%, and <90% as the first, second, and third, respectively. The percentage of area packed with coils was calculated with the algebraic equation : (1-area of contrast filling/area of aneurysm measured on working view)×100%. In every case, two experienced neurointerventionists, who were not informed of presence or absence of recanalization, determined both AGS and 2DPO after reviewing postprocedural angiograms, and consensus was reached by means of discussion in cases of discrepancy. Lastly, vPD was calculated by a neurointerventionist and stratified into three categories : >30%, 24-30% and <24% as the first, second, and third, respectively, using calculation methods previously described7,22) : aneurysm volume=4π (height/2×length/2×width/2)/3; coil volume=π (diameter of coil/2)2×length of coil; and vPD=(coil volume/aneurysm volume)×100%. Height, length and width of aneurysms were measured on 3D images.
When unruptured aneurysms were successfully embolized, MR angiography (MRA) was recommended at 6, 12, and 24 months postembolization. For ruptured aneurysms, fluoroscopic radiographs were obtained at 1 and 3 months postembolization in the procedural working projection, and MRA was examined at 6, 12, and 24 months. When significant coil compaction or major recanalization was strongly suspected in noninvasive studies, conventional angiography was performed immediately to assess the state of the aneurysm, and to determine the need for further treatment. Once stable occlusion was demonstrated angiographically, follow-up MRA was recommended after another 6, 12, and 24 months. In patients who underwent repeat embolization, MRA was also recommended 6, 12, and 24 months following repeat procedure. If the aneurysm occlusion was stable at 24 months after the initial or repeat treatment, follow-up would either be discontinued or continued until after 36 months, on a case-by-case basis.
Recanalization was defined as the presence of a new or increased intraaneurysmal flow on follow-up images. Major recanalization was defined as the significant recanalization that repeat treatment should be recommended. Patients were classified into two groups for analysis : presence or absence of major recanalization. Two neurointerventionists determined that 6 weeks after rating the AGS and 2DPO. Disagreements were resolved by consensus.
Baseline characteristics of patients and procedures were compared using the two-sample t-test or Mann-Whitney U test for continuous and ordinal data and chi-square test for categorical variables. Life-table and Kaplan-Meier analysis with log-rank test was used to calculate the cumulative risk (CR) of recanalization for each scale, and compare recanalization-free survival distribution for categories of each scale. Observations were censored when a patient was lost to follow-up. Age, sex, type of presentation (ruptured or unruptured), shape of aneurysm (lobulated or single saccular), maximal size of aneurysm, neck width, follow-up period, aneurysm location, and initial occlusion rates of each scale were evaluated individually by univariate Cox proportional hazards model. All variables significantly associated with recanalization (p<0.05) were included in a multivariable model. Then, we computed Harrell's concordance index (C-index) of each scale measuring initial occlusion rates3). The C-index was a measure of the predictive accuracy of a Cox proportional hazards model for each scale; a value of <0.5 indicates that the method cannot predict the recanalization of coiled aneurysm, while the maximum value of 1 indicates perfect prediction of aneurysmal recanalization. Statistical analyses were performed with the SAS 9.2 software for Windows (SAS institute, Cary, NC, USA).
During a mean of 17.6 months follow-up (range 5 to 72 months), major recanalization occurred in 63 of 730 aneurysms (8.6%). Comparing baseline characteristics of the recanalization group (RG) versus the non-recanalization group, age and gender were not significantly different. Ruptured presentation (39.7% versus 19.8%) and aneurysmal lobulation (73.0% versus 50.4%) were more common in the RG (p<0.05), and maximal size (8.89±3.47 mm versus 5.58±2.61 mm) and neck width (5.44±2.35 mm versus 3.52±1.35 mm) were larger in the RG (p<0.05). Comparison of baseline characteristics is summarized in Table 1.
The occlusion rates by AGS was assessed as the first category in 295 (40.4%), the second in 178 (24.4%), and the third in 257 (35.2%). Using 2DPO, the first category was observed in 295 (40.4%), the second in 172 (23.6%), and the third in 263 (36.0%). Using vPD, the first, second, and third categories were observed in 357 (48.9%), 194 (26.6%), and 179 (24.5%), respectively. There were 65 disagreements of initial occlusion rates between AGS and 2DPO. Thirty-six of 65 cases were in the second category of AGS and the third category of 2DPO (group D1), while the remaining 29 cases in the third category of AGS and the second category of 2DPO (group D2). Major recanalization in the group D1 and D2 (p=0.04 by Fisher's exact test) occurred in 2 (5.5%) and 7 (24.1%), respectively.
In life-table and Kaplan-Meier analysis, the CR of recanalization was 3% at 6 months, 7% at 12 months, 10% at 24 months, and 13% at 36 months after coil embolization. The CR of recanalization of the first category of AGS was 1%, 2%, 4%, and 4% at 6 months, 12 months, 24 months and 36 months, respectively; the second category of AGS was 6%, 9%, 15% and 15% at 6, 12, 24 and 36 months; the third category of AGS was 4%, 12%, 15%, 20% at 6, 12, 24, 36 months. The CR of recanalization of the first category of 2DPO was 1%, 3%, 4%, 4% at 6, 12, 24, 36 months; the second category of 2DPO was 8%, 13%, 15%, 33% at 6, 12, 24, 36 months; the third category of 2DPO was 3%, 9%, 14%, 14% at 6, 12, 24, 36 months. The CR of recanalization of the first category of vPD was 1%, 4%, 5%, 10% at 6, 12, 24, 36 months; the second category of vPD was 4%, 8%, 14%, 18% at 6, 12, 24, 36 months; the third category of vPD was 6%, 12%, 14%, 14% at 6, 12, 24, 36 months. The CR of recanalization was significantly higher in the less occluded categories of every scale (p<0.05). However, the risks in the second and third categories were not significantly different for AGS (p=0.93) and 2DPO (p=0.08), whereas it was significantly higher in the vPD's second category than its third, which was 18% versus 14% at 36 months (Fig. 1).
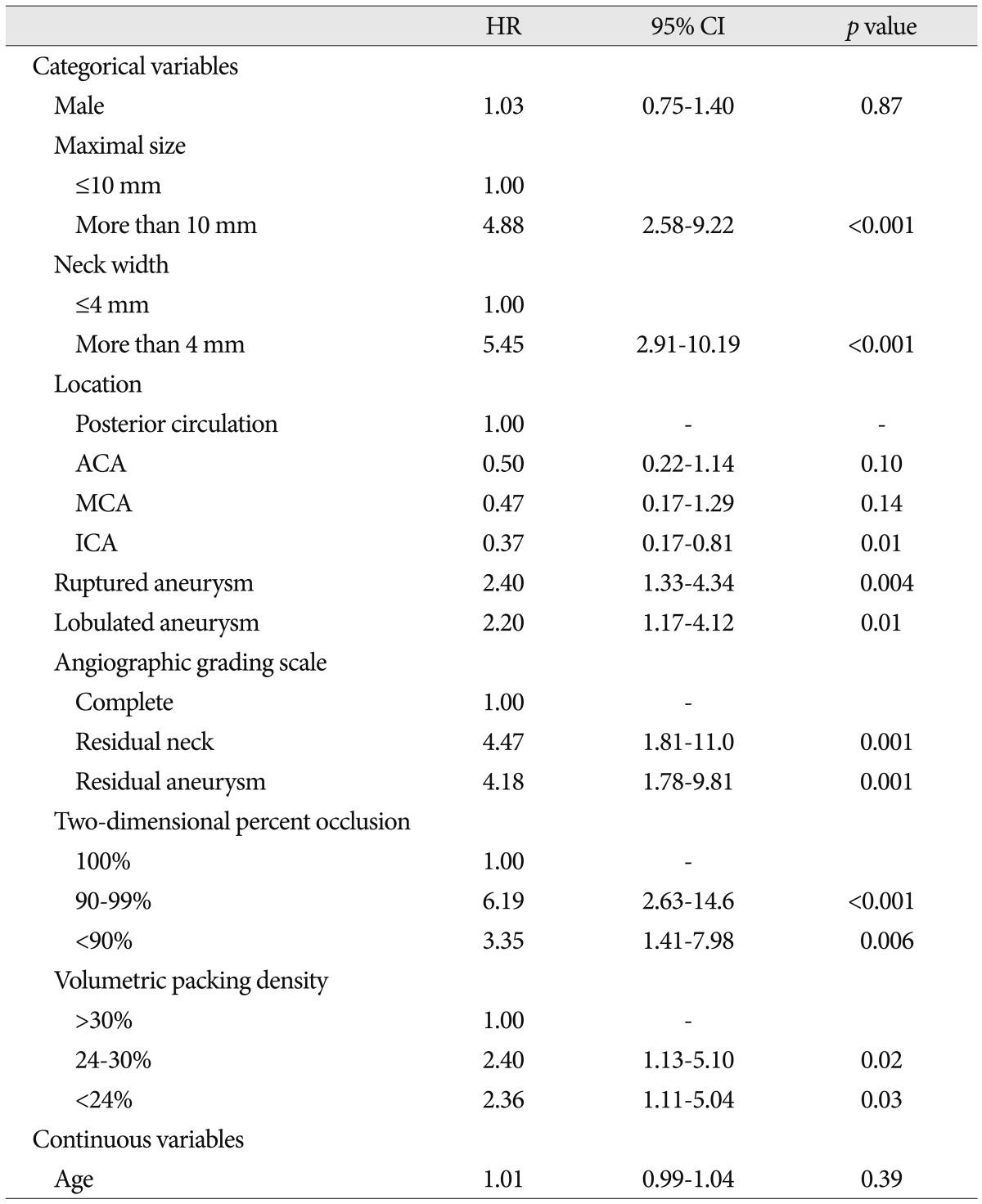
Table 2 and 3 show predictors of recanalization analyzed by Cox proportional hazards model. Maximal size, neck width, and AGS and 2DPO scales were associated with recanalization in a multivariable model including all potential predictors identified in univariate analysis. Ruptured presentation also had a significant relationship with recanalization after adjustment for any type of scales and other potential confounders. There was a stepwise increase in the risk of recanalization across all categories of AGS [the second category, hazard ratio (HR) : 3.95, 95% confidence interval (CI) : 1.57-9.96, p=0.004; the third category, HR : 4.15, 95% CI : 1.79-9.61, p=0.001]. 2DPO was also associated with the risk of recanalization, but the second category (90-99%) had slightly higher risk of recanalization than the third (HR : 4.87 vs. 3.12, 95% CI : 2.01-11.9 vs. 1.31-7.41, p≤0.001 vs. 0.01). The vPD was not associated with recanalization (p>0.05). The validated and optimism-adjusted C-indices were 0.50 (CI : -1.09-2.09), 0.47 (CI : -1.10-2.09) and 0.44 (CI : -1.10-2.08) for AGS, 2DPO and vPD, respectively.
The goal of aneurysm coiling is the prevention of rupture or rebleeding by occluding aneurysmal flow. However, some studies indicated that coiling in ruptured aneurysms is significantly limited by recanalization9,12,16,19), and the rate of rebleeding tends to be higher than surgical clipping5). The International Subarachnoid Aneurysm Trial investigators, however, suggested in their first report that the risk of rebleeding from treated aneurysms is low with either coiling or clipping, although somewhat more frequent with coiling10,11). Their study also reassured that the initial observed clinical benefits of coiling compared to clipping were not lost over time with the low rate of rebleeding11). Nevertheless, the issue of recanalization should not be overlooked in patients who underwent coiling for ruptured intracranial aneurysms. It is not surprising that the primary procedural outcome would be a significant predictor of recanalization. Several types of scales are being used to assess the occlusion rates but the definition and number of categories in each scale are variable. Moreover, the scales have never been formally validated. Consequently, it is hard to directly compare anatomic outcomes of relevant studies and conclude on evidence base.
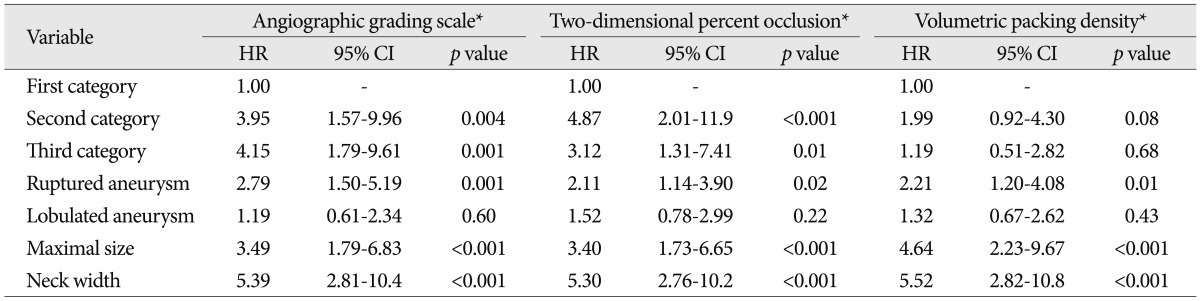
The present study showed that AGS was the most adequate to predict recanalization based on both the Cox proportional hazards model and the C-index. In addition, AGS has the merit of being easier to use because of its simple visual assessment. In terms of assessment details, only AGS reflects in-depth filling of contrast material in coiled aneurysms. In contrast, 2DPO introduces inherent errors in measuring the occlusion rates; being calculated as percent to an area of aneurysm at a specific two-dimensional projection, it can neither represent occlusion rate of the overall aneurysm nor reflect occlusion of the aneurysmal neck, which contains the inflow zone. In this respect, AGS may denote the potential site of recanalization because the presence of in-depth filling appears to be closely related to recanalization, especially at the inflow zone4,12,23). The fact that the rate of recanalization was higher in the group D2 than D1 would support this assumption. Therefore, the representation of occlusion rates as a percent seems not to be an adequate concept. With respect to the risk of recanalization, Kaplan-Meier curve showed a higher CR for both the second and third categories compared to the first one and HRs of the second and third categories were found to be similar for AGS and 2DPO. Therefore, we should keep in mind that a non-complete occlusion regardless of the category presents some risks of recanalization during follow-up.
Previously, several reports have showed that higher packing prevents coil compactions or recanalizations after endovascular coiling of cerebral aneurysms6,7,21,22). Their results suggested that the chance of recanalization would be very low if a packing density were more than 30%. A packing density of 20% and 24% has been proposed as a cutoff value of compaction in aneurysms with a volume of <200 mm3 and <600 mm3, respectively21). The present study, though the risk of recanalization was associated with the vPD categories in univariate regression analysis, showed that the increased risk in less occluded categories did not persist after adjustment for potential confounders. The vPD is calculated on the basis of 3-dimensinal volume unit, contrary to AGS and 2DPO, which is thought to render vPD measurement close to the real value by eliminating any errors due to subjective and 2-dimensional measurement. However, it appears that vPD has an inherent limitation in measuring aneurysmal volume that is based on ellipsoid estimation of an aneurysm. It would not provide an accurate aneurysmal volume because lobulation or irregularity of aneurysm is relatively common. Moreover, erroneous measurement is easy to occur when the aneurysm is very small, where a little change in a point of cursor in the 3D workstation would make a big difference in aneurysm volume and vPD. These potential inherent errors of vPD measurement and calculation may have caused the absence of significant association after multivariate adjustment between vPD and recanalization. To reduce these errors in measuring aneurysmal volume, a direct measurement function was added to the 3D workstation14). However, it is not also free from errors; manual outlining and segmentation of the aneurysm from the parent artery could vary from person to person; and different window settings for appropriate background noise and aneurysm delineation would present different aneurysmal volumes for a single aneurysm.
Aside from the general limitations of the retrospective study, there are other limitations in this study. As MRA was used to determine recanalization in most cases, the rate of recanalization may have been inaccurate. However, a retreatable aneurysm with recanalization would have been unlikely to be missed, having reviewed both maximum intensity projection images and raw data sets of 3D time-of-flight MR angiography. Moreover, it is well known that the use of MRA has high sensitivity and specificity for recanalization25). Some may criticize the three-tiered categorization of 2DPO (100%, 90-99%, and <90%) and vPD (>30%, 24-30%, and <24%) by artificial cut-off values. However, it is commonly used in practice, and additional statistical analyses of differently categorized data with varying cut-off values did not show any different meaningful results.
Most variables known to increase a risk of recanalization were repeatedly found to be associated with recanalization of coiled aneurysms. Of the three representative scales, AGS is likely to be the most adequate to predict recanalization and even most easy to assess. If an aneurysm was not completely occluded with coils, even second category occlusion would not ensure durability because the risk of recanalization is similarly high in the second and third categories. Lastly, vPD should not be used by itself to predict recanalization.
References
1. Cognard C, Weill A, Castaings L, Rey A, Moret J. Intracranial berry aneurysms : angiographic and clinical results after endovascular treatment. Radiology. 1998; 206:499–510. PMID: 9457205.

2. Gallas S, Pasco A, Cottier JP, Gabrillargues J, Drouineau J, Cognard C, et al. A multicenter study of 705 ruptured intracranial aneurysms treated with Guglielmi detachable coils. AJNR Am J Neuroradiol. 2005; 26:1723–1731. PMID: 16091521.
3. Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models : issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996; 15:361–387. PMID: 8668867.

4. Hayakawa M, Murayama Y, Duckwiler GR, Gobin YP, Guglielmi G, Viñuela F. Natural history of the neck remnant of a cerebral aneurysm treated with the Guglielmi detachable coil system. J Neurosurg. 2000; 93:561–568. PMID: 11014533.

5. Johnston SC, Dowd CF, Higashida RT, Lawton MT, Duckwiler GR, Gress DR. CARAT Investigators. Predictors of rehemorrhage after treatment of ruptured intracranial aneurysms : the Cerebral Aneurysm Rerupture After Treatment (CARAT) study. Stroke. 2008; 39:120–125. PMID: 18048860.

6. Kai Y, Hamada J, Morioka M, Yano S, Kuratsu J. Evaluation of the stability of small ruptured aneurysms with a small neck after embolization with Guglielmi detachable coils : correlation between coil packing ratio and coil compaction. Neurosurgery. 2005; 56:785–792. discussion 682-683. PMID: 15792517.

7. Kawanabe Y, Sadato A, Taki W, Hashimoto N. Endovascular occlusion of intracranial aneurysms with Guglielmi detachable coils : correlation between coil packing density and coil compaction. Acta Neurochir (Wien). 2001; 143:451–455. PMID: 11482694.

8. Kwon BJ, Chang HW, Youn SW, Kim JE, Han MH. Intracranial aneurysm perforation during endosaccular coiling : impact on clinical outcome, initial occlusion, and recanalization rates. Neurosurgery. 2008; 63:676–682. discussion 682-683. PMID: 18981878.
9. Mason AM, Cawley CM, Barrow DL. Surgical management of intracranial aneurysms in the endovascular era : review article. J Korean Neurosurg Soc. 2009; 45:133–142. PMID: 19352474.

10. Molyneux A, Kerr R, Stratton I, Sandercock P, Clarke M, Shrimpton J, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms : a randomised trial. Lancet. 2002; 360:1267–1274. PMID: 12414200.

11. Molyneux AJ, Kerr RS, Birks J, Ramzi N, Yarnold J, Sneade M, et al. Risk of recurrent subarachnoid haemorrhage, death, or dependence and standardised mortality ratios after clipping or coiling of an intracranial aneurysm in the International Subarachnoid Aneurysm Trial (ISAT) : long-term follow-up. Lancet Neurol. 2009; 8:427–433. PMID: 19329361.

12. Murayama Y, Nien YL, Duckwiler G, Gobin YP, Jahan R, Frazee J, et al. Guglielmi detachable coil embolization of cerebral aneurysms : 11 years' experience. J Neurosurg. 2003; 98:959–966. PMID: 12744354.

13. Niimi Y, Song J, Madrid M, Berenstein A. Endosaccular treatment of intracranial aneurysms using matrix coils : early experience and mid-term follow-up. Stroke. 2006; 37:1028–1032. PMID: 16514098.

14. Piotin M, Daghman B, Mounayer C, Spelle L, Moret J. Ellipsoid approximation versus 3D rotational angiography in the volumetric assessment of intracranial aneurysms. AJNR Am J Neuroradiol. 2006; 27:839–842. PMID: 16611775.
15. Piotin M, Spelle L, Mounayer C, Salles-Rezende MT, Giansante-Abud D, Vanzin-Santos R, et al. Intracranial aneurysms : treatment with bare platinum coils--aneurysm packing, complex coils, and angiographic recurrence. Radiology. 2007; 243:500–508. PMID: 17293572.

16. Raymond J, Guilbert F, Weill A, Georganos SA, Juravsky L, Lambert A, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke. 2003; 34:1398–1403. PMID: 12775880.

17. Roy D, Milot G, Raymond J. Endovascular treatment of unruptured aneurysms. Stroke. 2001; 32:1998–2004. PMID: 11546888.

18. Slob MJ, Sluzewski M, van Rooij WJ, Roks G, Rinkel GJ. Additional coiling of previously coiled cerebral aneurysms : clinical and angiographic results. AJNR Am J Neuroradiol. 2004; 25:1373–1376. PMID: 15466335.
19. Sluzewski M, van Rooij WJ, Rinkel GJ, Wijnalda D. Endovascular treatment of ruptured intracranial aneurysms with detachable coils : long-term clinical and serial angiographic results. Radiology. 2003; 227:720–724. PMID: 12773678.

20. Sluzewski M, van Rooij WJ, Beute GN, Nijssen PC. Late rebleeding of ruptured intracranial aneurysms treated with detachable coils. AJNR Am J Neuroradiol. 2005; 26:2542–2549. PMID: 16286399.
21. Sluzewski M, van Rooij WJ, Slob MJ, Bescós JO, Slump CH, Wijnalda D. Relation between aneurysm volume, packing, and compaction in 145 cerebral aneurysms treated with coils. Radiology. 2004; 231:653–658. PMID: 15118115.

22. Tamatani S, Ito Y, Abe H, Koike T, Takeuchi S, Tanaka R. Evaluation of the stability of aneurysms after embolization using detachable coils : correlation between stability of aneurysms and embolized volume of aneurysms. AJNR Am J Neuroradiol. 2002; 23:762–767. PMID: 12006273.
23. Tateshima S, Murayama Y, Gobin YP, Duckwiler GR, Guglielmi G, Viñuela F. Endovascular treatment of basilar tip aneurysms using Guglielmi detachable coils : anatomic and clinical outcomes in 73 patients from a single institution. Neurosurgery. 2000; 47:1332–1339. discussion 1339-1342. PMID: 11126904.
24. Thornton J, Debrun GM, Aletich VA, Bashir Q, Charbel FT, Ausman J. Follow-up angiography of intracranial aneurysms treated with endovascular placement of Guglielmi detachable coils. Neurosurgery. 2002; 50:239–249. discussion 249-250. PMID: 11844258.

25. Wallace RC, Karis JP, Partovi S, Fiorella D. Noninvasive imaging of treated cerebral aneurysms, part I : MR angiographic follow-up of coiled aneurysms. AJNR Am J Neuroradiol. 2007; 28:1001–1008. PMID: 17569946.

Fig. 1
Kaplan-Meier analyses demonstrate the proportion of patients without recanalization during follow-up. Kaplan-Meier plot of angiographic grading scale (A), two-dimensional percent occlusion (B), and volumetric packing density (C).
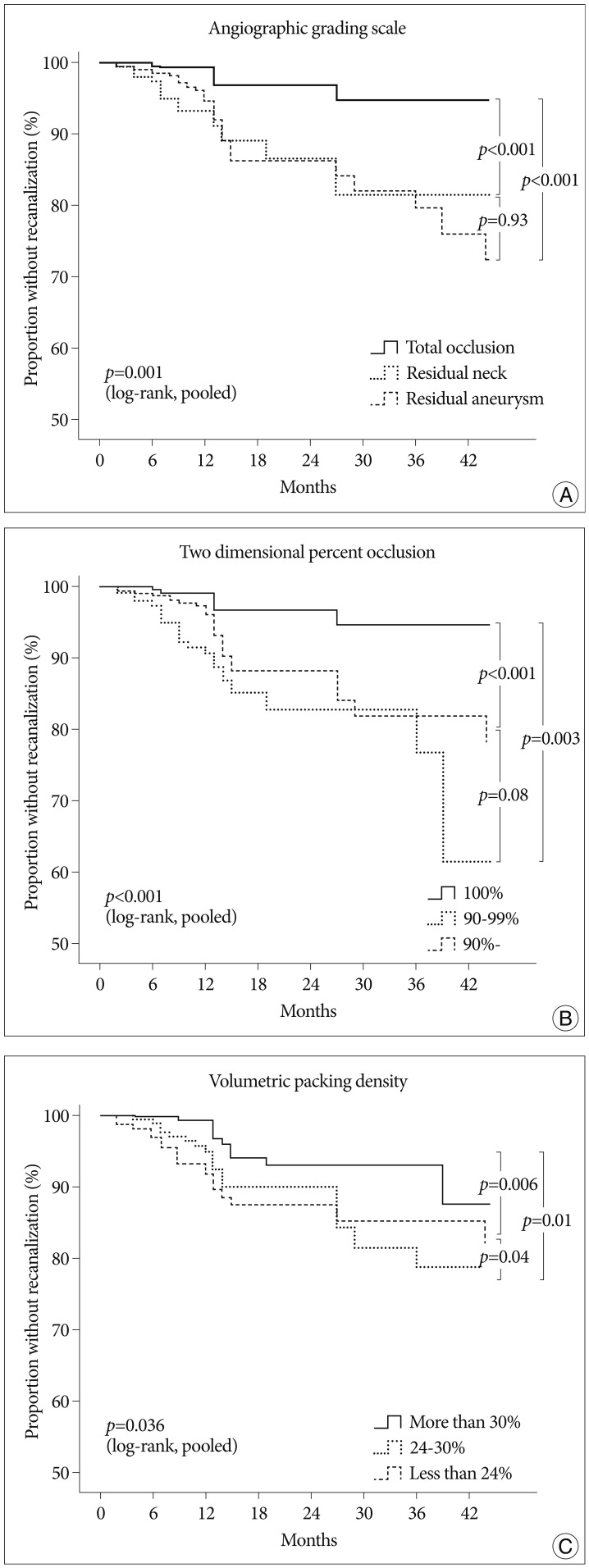
Table 1
Baseline characteristics
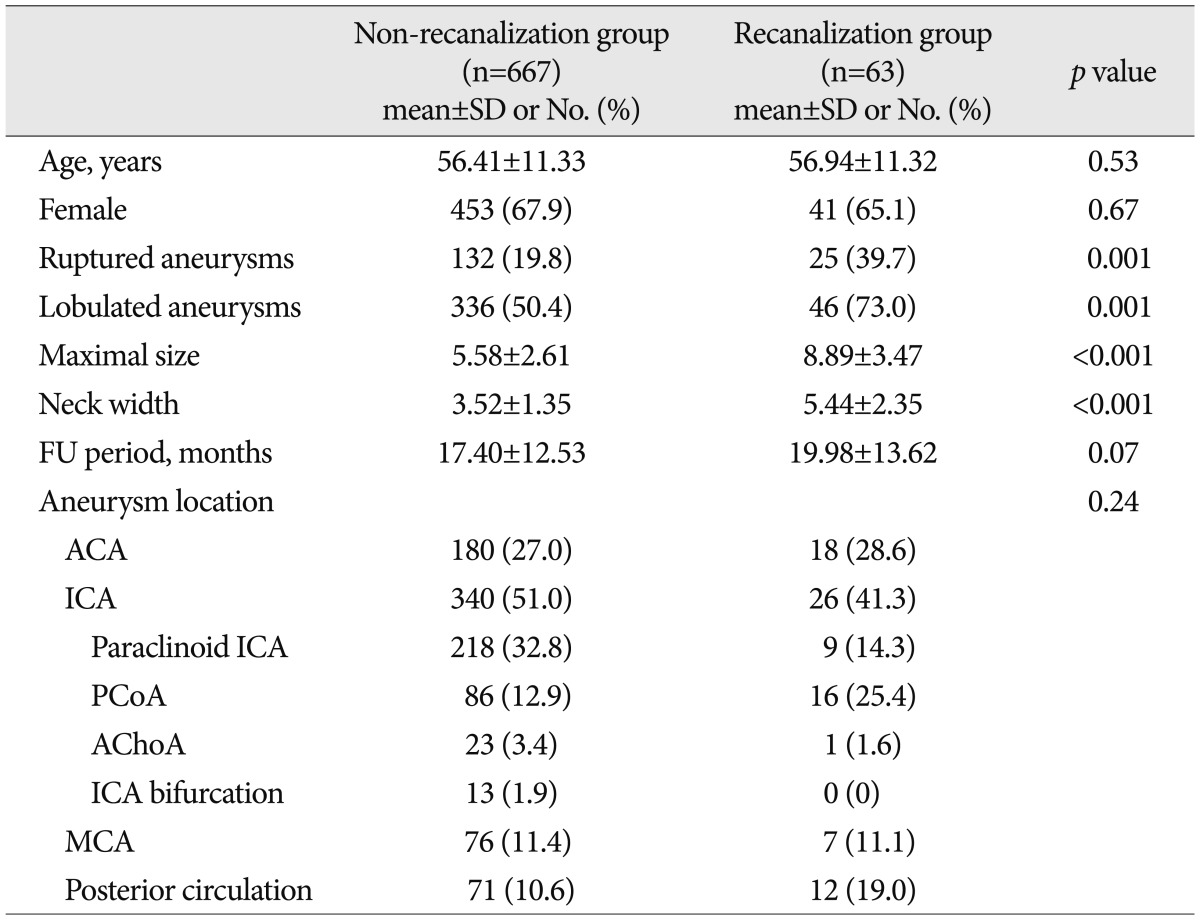
p-value was indicated by two-sample t-test or Mann-Whitney U test for continuous variables and chi-square test for categorical variables. Numbers in parentheses are percentages. FU : follow-up, ACA : anterior cerebral artery, ICA : internal cerebral artery, MCA : middle cerebral artery, PCoA : posterior communicating artery, AChoA : anterior choroidal artery, SD : standard deviation




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download