Abstract
Fusiform aneurysms on the basilar artery (BA) trunk are rare. The microsurgical management of these aneurysms is difficult because of their deep location, dense collection of vital cranial nerves, and perforating arteries to the brain stem. Endovascular treatment is relatively easier and safer compared with microsurgical treatment. Selective occlusion of the aneurysmal sac with preservation of the parent artery is the endovascular treatment of choice. But, some cases, particularly giant or fusiform aneurysms, are unsuitable for selective sac occlusion. Therefore, endovascular coiling of the aneurysm with parent vessel occlusion is an alternative treatment option. In this situation, it is important to determine whether a patient can tolerate parent vessel occlusion without developing neurological deficits. We report a rare case of fusiform aneurysms in the BA trunk. An 18-year-old female suffered a headache for 2 weeks. Computed tomography and magnetic resonance image revealed a fusiform aneurysm of the lower basilar artery trunk. Digital subtraction angiography revealed a 7.1×11.0 mm-sized fusiform aneurysm located between vertebrovasilar junction and the anterior inferior cerebellar arteries. We had good clinical result using endovascular coiling of unruptured fusiform aneurysm on the lower BA trunk with parent vessel occlusion after confirming the tolerance of the patient by balloon test occlusion with induced hypotension and accompanied by neurophysiologic monitoring, transcranial Doppler and single photon emission computed tomography. In this study, we discuss the importance of preoperative meticulous studies for avoidance of delayed neurological deficit in the patient with fusiform aneurysm on lower basilar trunk.
Fusiform aneurysms on the basilar artery (BA) trunk are rare17,24). The microsurgical management of these aneurysms is difficult because of their deep location, dense collection of vital cranial nerves, and perforating arteries to the brain stem9). The endovascular approach is comparatively easy and comfortably compares with surgical treatment. Thus, endovascular treatment is considered as first treatment option in these lesions. Selective occlusion of the aneurysmal sac with preservation of the parent artery is the treatment of choice8). But, some cases, particularly giant or fusiform aneurysms, are unsuitable for selective sac occlusion2,6,15). Therefore, proximal occlusion of the parent vessel is an alternative treatment option. It is important to determine whether a patient can tolerate proximal occlusion without developing neurological deficits.
We report a rare case of fusiform aneurysms in the BA trunk that was treated successfully with endovascular method after confirming bilateral collateral bloods flow through the both posterior communicating arteries, the tolerance of the patient by balloon test occlusion (BTO) with induced hypotension and accompanied by neurophysiologic monitoring (NPM), transcranial Doppler (TCD) and single photon emission computed tomography (SPECT).
An 18-year-old female suffered a headache for 2 weeks. Computed tomography and magnetic resonance image revealed a fusiform aneurysm on the lower BA trunk. Digital subtraction angiography (DSA) revealed a 7.1×11.0 mm-sized fusiform aneurysm located just below the anterior inferior cerebellar artery (AICA) (Fig. 1). Right side posterior inferior cerebellar artery was not seen. Both posterior cerebral arteries (PCA) and superior cerebellar arteries (SCA) filled normally. Both posterior communicating arteries (PComA) were patent and the diameter of both arteries was more than 1 mm in diameter (Fig. 1). For the proximal portion of the aneurysm forming a severe acute angle, flow diversion with multiple stent is difficult to enforce. Endovascular coiling of aneurysm with occlusion of dysplastic parent vessel is planned if the patient tolerate complete preoperative occlusion test.
The location of aneurysm is just distal to vertebrobasilar junction and proximal to the origin of AICA, and it is somewhat related perforating artery injury to perform a BTO at basilar artery. We planned the BTO on both vertebral arteries. BTO was performed under local anesthesia and accessible for neurological examination. At the time of the BTO, the neurological status of the patient was continuously monitored by a trained neurosurgeon that assessed speech, motor strength, sensory extinction, and level of alertness (Fig. 2).
Bilateral femoral artery (FA) punctures were performed and guide catheter placed in both vertebral arteries (VA). Additional right FA puncture was performed for diagnostic cerebral angiogram. The patient was anticoagulated by the intravenous injection of 5000 IU heparin. Over-the-wire microballoon (Hyperfrom, Hyperglide; ev3, Irvine, CA, USA) was used for BTO and these balloons were located at VA just around the C2 vertebra foramen (Fig. 2). The balloon was then inflated. Complete balloon occlusion of VA was verified by a proximal contrast injection. The BA and both AICAs were visible through the retrograde blood flow from the PComA, which were visualized by an angiographic injection in the both internal carotid artery (ICA).
Bilateral BTO of the each VA were performed over 20 min under a normotensive state. Baseline blood pressure was 109/72 mm Hg (mean arterial blood pressure, 84.33 mm Hg). The patient tolerated 20 minutes under the normotensive state and hypotension was induced pharmacologically by the intravenous infusion of nicardipine (starting dose of 5 mg/hr with 2.5 mg/hr increase every 5 min if the blood pressure did not reach the target range). After the mean arterial pressure was reduced by 20% of baseline (92/56 mm Hg; mean arterial blood pressure, 68.0 mm Hg), hypotension was maintained for 20 minutes.
Before BTO, the patient underwent a complete TCD investigation. The patient-customized TCD headset was mounted. BTO was performed with concomitant monitoring of the blood velocity and direction of the blood flow in the middle cerebral artery (MCA) and P1 segment of the PCA.
DSA during BTO, the right ICA angiogram showed retrograde filling of the distal part of the BA, PCAs, SCAs and AICASs through the PComA (Fig. 2). The patient tolerated 20 minutes under the normotensive state and induced hypotensive state with no developing neurological deficits.
Baseline mean blood flow velocity of MCA was 74 cm/s (Fig. 3). The velocity during BTO dropped immediately to 48 cm/s upon balloon inflation, but returned to the baseline value (72 cm/s) within several seconds without additional treatment and was maintained during the BTO. After induced hypotension, it dropped to 65 cm/s; the patient tolerated in induced hypotensive condition. After deflation of the balloon, the velocity of the MCA recovered to the baseline value. The reduction of flow velocity was 2.7% under the normotensive state and 12.2% under induced hypotensive state.
Baseline SPECT images did not show any focal perfusion reduction (Fig. 4). SPECT images obtained after BTO also did not showed hypoperfusion of the cerebral hemisphere and cerebellum compare to the baseline SPECT.
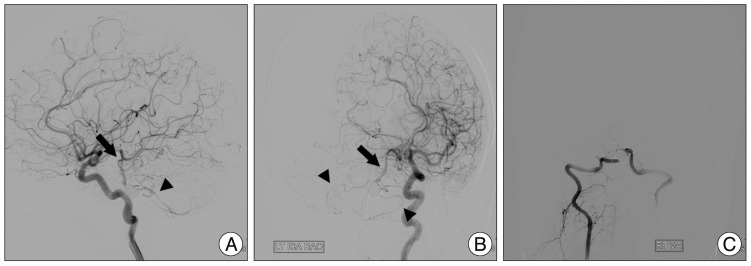
Coil embolization was performed with multiple coils in the usual way. Care was taken to preserve the both AICAs, which arose close to the distal aspect of the aneurysm. After coil embolization, a left side ICA angiogram (Fig. 5) showed regrograde filling of the BA (arrow) and both AICAs (arrowhead) through the PComA. A right side VA angiogram showed complete obliteration of the aneurysm and no filling of the BA though the VA.
Fusiform aneurysms in the BA trunk are rare17,24) and their prognosis is generally bleak. Left untreated, progressive brain-stem compression or subarachnoid hemorrhage may result, with survival as low as 20% after 2 years4,14). Unfortunately, the surgical treatment is very difficult because of the deep location of the aneurysm, dense collection of vital cranial nerves, and perforating arteries to the brain stem9). However, the endovascular approach to this area is comparatively easy, so the first choice of treatment has changed over the last 10 years7,23,25). Selective occlusion of the aneurysmal sac, with preservation of the parent artery, is the treatment of choice8). But, some cases, particularly giant or fusiform aneurysms, are unsuitable for selective sac occlusion2,6,15). In these circumstances, proximal occlusion of the parent vessel is an alternative treatment option. It is important to determine before the operation whether a patient can tolerate arterial occlusion without developing neurological deficits. Although there is no way to guarantee absence of complications after the occlusion of the parent vessel, various preoperative occlusion tests for the selection of patients who can tolerate the loss of the parent artery have been developed.
Tolerance of the proximal arterial occlusion depends on collateral flow to distal branches. The diameter of the PComA is an important predictor of success of basilar occlusion4,16,20). Steinberg et al.20) reported that a PComA diameter greater than 1 mm is significantly related to tolerance of BA occlusion, and a favorable long-term clinical outcome. But, in one study, 8% of patients with two large PComAs (both posterior communicating arteries exceed 1 mm) experienced ischemic complications20). Pelz et al.16) reported a trend in which patients with a large PComA will have a better clinical outcome than patients without a large PComA, but the finding was not statistically significant. Also, retrograde filling of the BA by collateral flow from the anterior circulation is an essential factor for parent artery occlusion.
Other predictors of tolerance include normal baseline cerebral blood flow and tolerance of balloon occlusion1,10,11,19). Linskey et al.11) conducted a systematic review of 254 patients in five studies in which an ICA was therapeutically sacrificed without BTO, and reported an average stroke rate of 26% and mortality rate of 12%. This contrasted with a review of 262 patients in eight studies in which the ICA occluded after performed a BTO with an average stroke rate of 13% and mortality rate of 3%. This reduction in stroke and death rate reached statistical significance, but the complication rate was still high. Also, the false negative rate of the normotensive BTO was about 5%19). To reduce the complication rate, hypotension-induced challenge was introduced19). When the vessel is occluded, and no deficit occurs in a normotensive patient, the blood pressure is pharmacologically lowered to a target pressure. Standard et al.19) reported that parent artery sacrifice in 19 patients after a clinically tolerated hypotensive BTO resulted in no hemodynamic ischemic infarction.
Sonographic evaluation of the MCA is obtained before and after balloon inflation. Mean blood flow velocity that does not decrease more than 30% is highly predictive of tolerance to carotid occlusion5). Reversal of flow in the P1 indicates a likelihood to tolerate the BTO in cases of basilar occlusion18).
The 99m Tc-HMPAO SPECT procedure is an alternative indicator22,26). In this approach, 99m Tc-HMPAO is injected intravenously 10 min after the balloon is inflated. After the BTO is completed, SPECT scanning shows activity from the tracer, and asymmetry or difference of the baseline study is detected qualitatively by visual inspection of the scan. Universally, it does not allow for accurate quantitative measurement of the cerebral blood flow (CBF). But, the brain perfusion index can be converted to a mean CBF value13). A decrease of more than 10% of regional CBF in the BTO study compared with the baseline study is considered to be significant enough to take additional measure of bypass grafting before performing the occlusion26).
Continuous electroencephalography monitoring is done throughout the procedures. Slowing or other deviation from baseline conditions can be secondary signs of developing ischemia3). Somatosensory evoked potential and brainstem auditory evoked potential monitoring can be tested. The cortical responses are recorded and the timing and amplitude of the response indicated cortical function21).
The main criteria for success with BTO are clinical neurological testing. In addition to simple neurological testing during BTO, a battery of standardized neuropsychological tests are given to test higher cortical functions12).
We report a rare case of fusiform aneurysm on BA trunk treated with endovascular coiling of the aneurysm with parent vessel occlusion after complete preoperative occlusion test. This method may provide an efficient and safe treatment modality. But, it can potentially lead to postoperative ischemic complications. Prior to the procedure, it requires meticulous evaluation of the aneurysm morphology and collateral pathway, multiple tests to predict hemodynamic state, and a provocation test to achieve a complete and safe result before the parent artery occlusion.
References
1. Aymard A, Gobin YP, Hodes JE, Bien S, Rüfenacht D, Reizine D, et al. Endovascular occlusion of vertebral arteries in the treatment of unclippable vertebrobasilar aneurysms. J Neurosurg. 1991; 74:393–398. PMID: 1993904.

2. Boardman P, Byrne JV. Giant fusiform basilar artery aneurysm : endovascular treatment by flow reversal in the basilar artery. Br J Radiol. 1998; 71:332–335. PMID: 9616247.

3. Cloughesy TF, Nuwer MR, Hoch D, Vinuela F, Duckwiler G, Martin N. Monitoring carotid test occlusions with continuous EEG and clinical examination. J Clin Neurophysiol. 1993; 10:363–369. PMID: 8408601.

4. Drake CG, Peerless SJ. Giant fusiform intracranial aneurysms : review of 120 patients treated surgically from 1965 to 1992. J Neurosurg. 1997; 87:141–162. PMID: 9254076.

5. Eckert B, Thie A, Carvajal M, Groden C, Zeumer H. Predicting hemodynamic ischemia by transcranial Doppler monitoring during therapeutic balloon occlusion of the internal carotid artery. AJNR Am J Neuroradiol. 1998; 19:577–582. PMID: 9541322.
6. Hassan T, Ezura M, Takahashi A. Treatment of giant fusiform aneurysms of the basilar trunk with intra-aneurysmal and basilar artery coil embolization. Surg Neurol. 2004; 62:455–462. discussion 462. PMID: 15518857.

7. Higa T, Ujiie H, Kato K, Kamiyama H, Hori T. Basilar artery trunk saccular aneurysms : morphological characteristics and management. Neurosurg Rev. 2009; 32:181–191. discussion 191. PMID: 18791752.

8. Higashida RT, Halbach VV, Cahan LD, Hieshima GB, Konishi Y. Detachable balloon embolization therapy of posterior circulation intracranial aneurysms. J Neurosurg. 1989; 71:512–519. PMID: 2795171.

9. Lawton MT, Daspit CP, Spetzler RF. Technical aspects and recent trends in the management of large and giant midbasilar artery aneurysms. Neurosurgery. 1997; 41:513–520. discussion 520-521. PMID: 9310966.

10. Lesley WS, Bieneman BK, Dalsania HJ. Selective use of the paraophthalmic balloon test occlusion (BTO) to identify a false-negative subset of the cervical carotid BTO. Minim Invasive Neurosurg. 2006; 49:34–36. PMID: 16547880.

11. Linskey ME, Jungreis CA, Yonas H, Hirsch WL Jr, Sekhar LN, Horton JA, et al. Stroke risk after abrupt internal carotid artery sacrifice : accuracy of preoperative assessment with balloon test occlusion and stable xenon-enhanced CT. AJNR Am J Neuroradiol. 1994; 15:829–843. PMID: 8059649.
12. Marshall RS, Lazar RM, Mohr JP, Pile-Spellman J, Hacein-Bey L, Duong DH, et al. Higher cerebral function and hemispheric blood flow during awake carotid artery balloon test occlusions. J Neurol Neurosurg Psychiatry. 1999; 66:734–738. PMID: 10329746.

13. Matsuda H, Yagishita A, Tsuji S, Hisada K. A quantitative approach to technetium-99m ethyl cysteinate dimer : a comparison with technetium-99m hexamethylpropylene amine oxime. Eur J Nucl Med. 1995; 22:633–637. PMID: 7498224.

14. Michael WF. Posterior fossa aneurysms simulating tumours. J Neurol Neurosurg Psychiatry. 1974; 37:218–223. PMID: 4819910.

15. Omahen DA, Findlay JM. A giant fusiform basilar aneurysm treated by bilateral vertebral artery occlusion. J Clin Neurosci. 2004; 11:324–328. PMID: 14975432.

16. Pelz DM, Viñuela F, Fox AJ, Drake CG. Vertebrobasilar occlusion therapy of giant aneurysms. Significance of angiographic morphology of the posterior communicating arteries. J Neurosurg. 1984; 60:560–565. PMID: 6699698.
17. Pia HW. Classification of vertebro-basilar aneurysms. Acta Neurochir (Wien). 1979; 47:3–30. PMID: 474201.

18. Sorteberg A, Bakke SJ, Boysen M, Sorteberg W. Angiographic balloon test occlusion and therapeutic sacrifice of major arteries to the brain. Neurosurgery. 2008; 63:651–660. dicussion 660-661. PMID: 18824944.

19. Standard SC, Ahuja A, Guterman LR, Chavis TD, Gibbons KJ, Barth AP, et al. Balloon test occlusion of the internal carotid artery with hypotensive challenge. AJNR Am J Neuroradiol. 1995; 16:1453–1458. PMID: 7484632.
20. Steinberg GK, Drake CG, Peerless SJ. Deliberate basilar or vertebral artery occlusion in the treatment of intracranial aneurysms. Immediate results and long-term outcome in 201 patients. J Neurosurg. 1993; 79:161–173. PMID: 8331396.

21. Su CC, Watanabe T, Yoshimoto T, Ogawa A, Ichige A. Proximal clipping of dissecting intracranial vertebral aneurysm--effect of balloon Matas test with neurophysiological monitoring. Case report. Acta Neurochir (Wien). 1990; 104:59–63. PMID: 2386090.

22. Tomura N, Omachi K, Takahashi S, Sakuma I, Otani T, Watarai J, et al. Comparison of technetium Tc 99m hexamethylpropyleneamine oxime single-photon emission tomograph with stump pressure during the balloon occlusion test of the internal carotid artery. AJNR Am J Neuroradiol. 2005; 26:1937–1942. PMID: 16155138.
23. Uda K, Murayama Y, Gobin YP, Duckwiler GR, Viñuela F. Endovascular treatment of basilar artery trunk aneurysms with Guglielmi detachable coils : clinical experience with 41 aneurysms in 39 patients. J Neurosurg. 2001; 95:624–632. PMID: 11596957.

24. Van Rooij WJ, Sluzewski M, Menovsky T, Wijnalda D. Coiling of saccular basilar trunk aneurysms. Neuroradiology. 2003; 45:19–21. PMID: 12525949.

25. Wang Q, Leng B, Song D, Chen G. Fusiform aneurysms of the vertebrobasilar arterial trunk : choice of endovascular methods and therapeutic efficacy. Acta Neurochir (Wien). 2010; 152:1467–1476. PMID: 20496084.

26. Yamamoto Y, Nishiyama Y, Toyama Y, Satoh K, Irie K, Ohkawa M. Preliminary results of Tc-99m ECD SPECT to evaluate cerebral collateral circulation during balloon test occlusion. Clin Nucl Med. 2002; 27:633–637. PMID: 12192280.

Fig. 1
A and B : Preoperative vertebral angiogram revealing fusiform aneurysm on the basialar artery trunk, located between the vertebrobasilar juction and anterior inferior cerebellar artery (arrow). C : Three dimensional angiogram reveals a 7.1×11.0 mm-sized fusiform aneurysm. D and E : Both internal carotid angiograms showed that both posterior communicating arteries were patent and the diameters exceeded 1 mm (arrowhead).
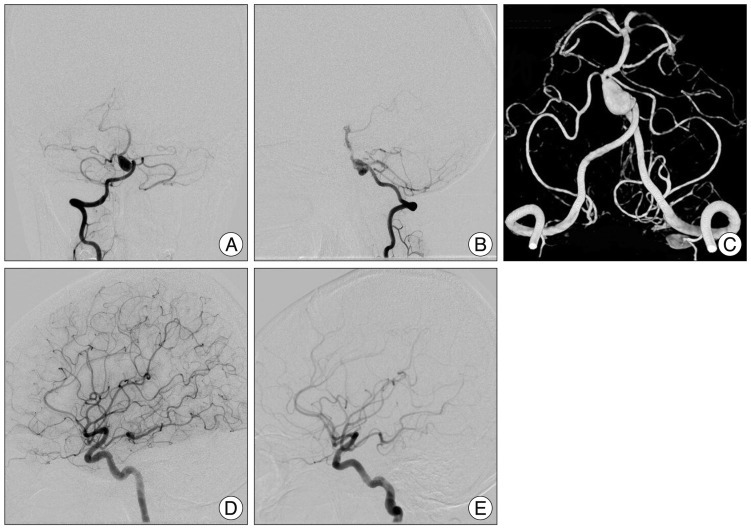
Fig. 2
A : The patient-customized transcranial Doppler headset was mounted and continuous electroencephalography monitoring was done throughout the procedure. B : Over-the-wire microballoons were used for balloon test occlusion and inflated in the both vertebral arteries just around the C2 vertebra foramen (arrow). C : Views of left side internal carotid angiograms. The basilar artery (black arrowhead) and both anterior inferior cerebellar arteries (white arrowhead) through the posterior communicating arteries are visualized by an angiographic injection in the left internal carotid artery.
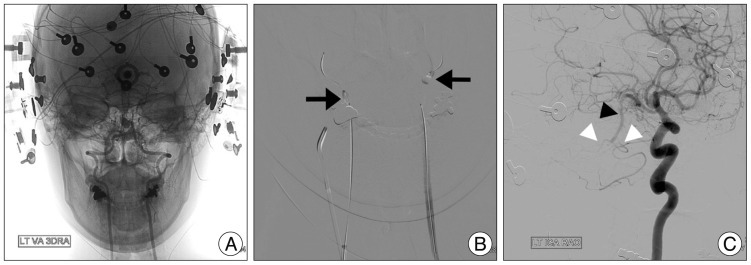
Fig. 3
A : Baseline mean blood flow velocity (MBFV) of middle cerebral artery (MCA) was 74 cm/s. B : The reduction in right MBFV of MCA (48 cm/s) and P1 reversal upon inflation of the balloon in both vertebral arteries. C : It immediately recovered at the baseline value (72 cm/s) without additional treatment. And it maintained during the balloon test occlusion. D : After induced hypotension the MBFV of the MCA slightly dropped (65 cm/s); the patient tolerated the drop during the test. E : Lastly, after deflation of the balloon it recovered to the baseline value (72 cm/s). The reduction of MBFV was 2.7% in the normotensive state and 12.2% in the induced hypotensive state.
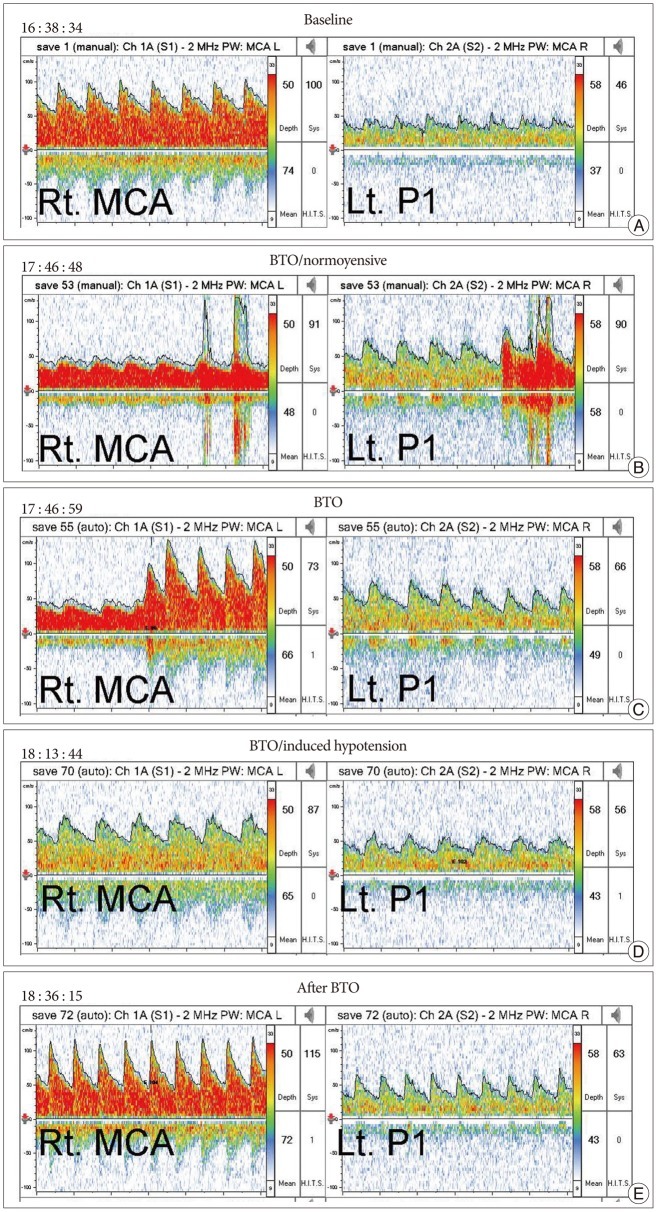
Fig. 4
A : Baseline single photon emission computed tomography (SPECT) images do not show focal perfusion reduction. B : SPECT images obtained during balloon test occlusion also do not show any hypoperfusion lesion of the cerebral hemisphere and cerebellum compare to the baseline SPECT.
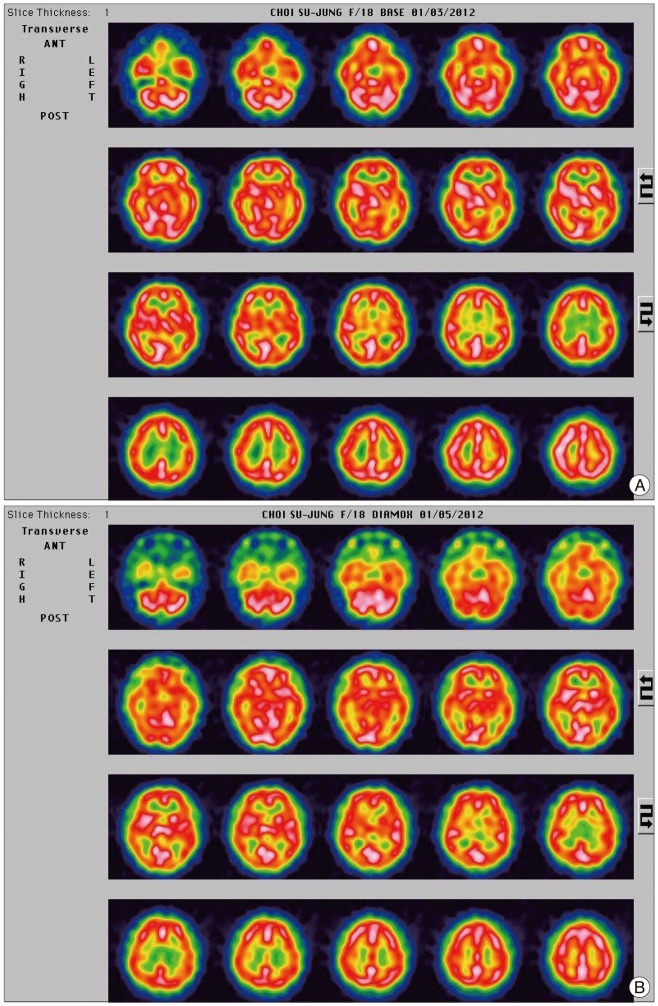
Fig. 5
A and B : After coil embolization, left side internal carotid artery angiogram reveals good filling of the basilar artery (arrow) and both anterior inferior cerebellar arteries (arrowhead) through the posterior communicating arteries. C : On right side vertebral artery angiogram shows complete obliteration of the aneurysm and no filling of the basilar artery though the vertebral artery.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download