Abstract
Granular cell tumors (GrCTs) of the spinal cord are rare benign tumors with a high rate of local recurrence. Only 6 cases of spinal GrCTs have been reported. GrCT is difficult to distinguish from other benign tumors such as schwannoma using imaging. A radiological "speckled dots" sign may be a useful differentiating feature of GrCT based upon experience with two cases and a review of the literature.
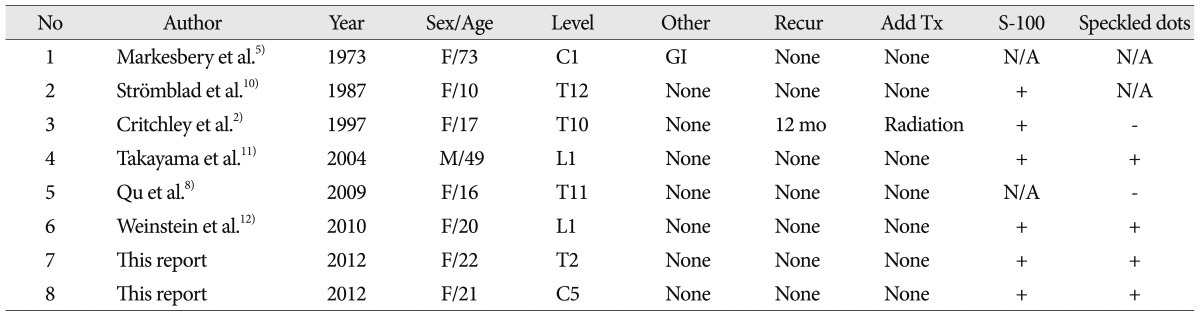
Granular cell tumors (GrCTs) are soft tissue tumors that are thought to originate from neural tissue based on immunohistochemical features. GrCTs can affect any part of the body but are most commonly found in the skin, tongue, ovaries, brain, and breast tissue5,6,9). Spinal intradural extramedullary (IDEM) GrCTs are exceptionally rare, and only 6 cases have been reported in the literature2,5,8,10-12).
GrCTs are usually benign tumors, and only 1-10% of GrCTs show malignant features3). However, the recurrence rate of benign GrCTs is reported to be as great as 20%, and that of malignant GrCTs is above 30%. Therefore, it is important to distinguish GrCTs from other benign tumors such as schwannomas because of the different clinical courses. Unfortunately, there are only a few case reports of GrCTs and no radiological differential points. The purpose of this report is to investigate the radiological characteristics of GrCT, with a review of reported cases, including ours.
A 22-year-old woman was admitted to the outpatient department with a 1-month history of left leg weakness and hypesthesia below the T2 level. Magnetic resonance (MR) images showed a 2.2×1.3 cm tumor at T1-2 (Fig. 1). The margin of the tumor was well defined, and the neural foramen was widened by the tumor, suggestive of a slow-growing tumor. The tumor showed broad isointensity with respect to the spinal cord on T1- and T2-weighted MR images and demonstrated homogeneous enhancement of the tumor on T1-weighted images. The tumor demonstrated an unusual pattern of internal low signal speckled dots on T1 enhanced MR images (Fig. 1E).
The patient underwent T1-2 laminoplastic laminotomy, and the tumor was exposed. The tumor looked similar to a typical schwannoma, appearing pearly yellow and white, with eccentric attachment to a nerve root. Although the tumor was friable, its capsule was tough and adhered tightly to the root. The two roots adhering to the capsule were sacrificed. At the completion of the resection, there was no change in the electrophysiological monitoring. On the histological exam, the tumor cell had abundant cytoplasm with eosinophilic granules (Fig. 2). Immunohistochemical analysis showed the tumor cells were strongly and diffusely immunoreactive with S-100 protein and CD68. The patient's hypesthesia improved after surgery. Follow-up MR imaging performed 12 months after surgery showed no evidence of recurrence.
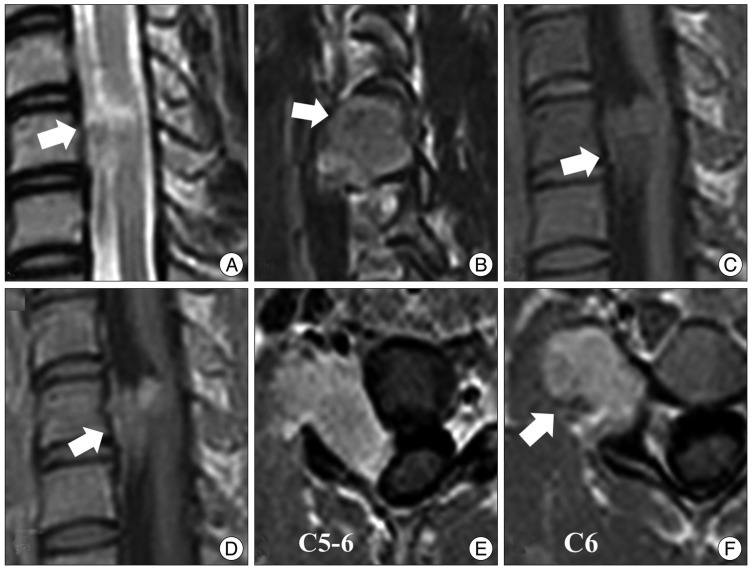
A 21-year-old woman presented with a 3-month history of right arm pain and tingling. The sensory changes had been present for 10 years, but she had not received any treatment. Her symptoms were aggravated after a minor car accident. She had no relevant medical or family history. Physical examination revealed intact motor power and normal reflexes. Computed tomography (CT) showed bony erosion at the right C5 and C6 foramina, and the right vertebral artery was displaced anteriorly. MR images demonstrated a 3.5×2.3 cm dumbbell-shaped IDEM mass at C5-6 that extended to the right extraforaminal space. The tumor showed isointensity with respect to the spinal cord on T1-weighted images and homogeneous gadolinium-enhanced T1-weighted images (Fig. 3). Speckled dots were also observed on the T2-weighed images and gadolinium enhanced T1-weighed images. As in Case 1, the tumor showed low signal speckled dots especially on the T1 enhanced images. Complete tumor resection was performed. The histological findings showed lymphocytic infiltration around granular cells (Fig. 2). The tumor cells were strongly immunoreactive with S-100 protein on the immunohistochemical analysis. The postoperative course was uneventful, and the patient experienced significant relief of her tingling sensation.
Our two GrCTs showed speckled dots in the tumor characterized by low signal dots, especially on enhanced T1-weighed images. We reviewed all of the published literature on GrCTs and found that the majority of published cases that included MR images also revealed this feature (Table 1). We have not observed such a pattern in other similar tumors, such as schwannoma and meningioma, nor has it been reported. Based on these results, speckled dots potentially are a characteristic for the differential diagnosis of GrCT.
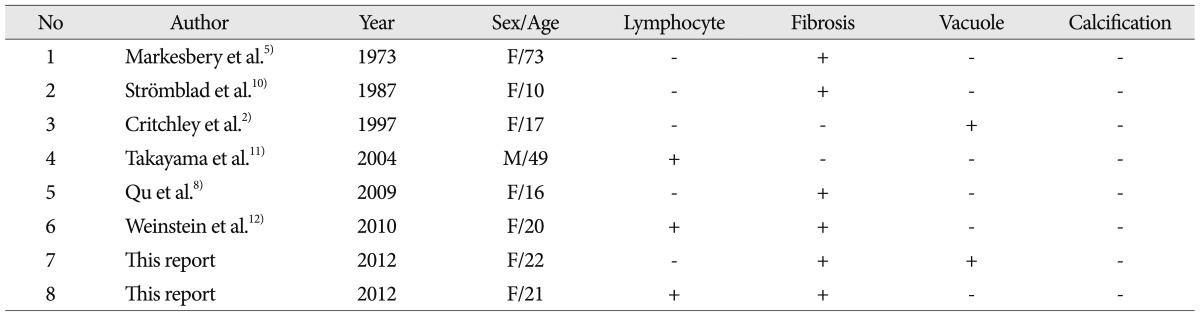
The physical reason behind the low signal speckled dots may be hyalinizing fibrosis of GrCTs. By histology, GrCTs consist of clusters and sheets of large rounded, polygonal or elongated cells with indistinct cellular borders. The tumor clusters consist of small, hyperchromatic to vesicular nuclei and background fibrous connective tissue. The islands of the tumors are separated by delicate fibrovascular tissue12). Case 1 showed fibrosis and vacuole changes, and Case 2 demonstrated hyalinizing fibrosis and lymphocytic infiltration. Other reported cases are summarized in Table 2. Fibrosis of GrCT is well described in 6 of 8 patients. The other 2 cases did not show fibrosis, but this was uncertain. Previous researchers have reported that GrCTs in the appendix and skin also demonstrate a telangiectatic portion with a fibrotic or hyalinized wall, stromal area and lymphoid infiltration7). Fibrosis may be a common factor correlating with the low signal intensities in MR. Therefore, speckled dots may be observed in GrCT as a result of the fibrosis.
It is believed that GrCT arises from Schwann cells1). This is supported by positive reactions to various neural markers, including S-100 protein, neuron-specific enolase, and inhibin-alpha3). One of our cases had a dumbbell-shaped tumor that appeared to have an intradural extramedullary origin, possibly from a spinal root. Both GrCT and schwannoma tended to occur more frequently at thoracolumbar junctions. These features would seem to indicate a close relation between GrCT and schwannoma. On the other hand, GrCTs can show malignant characteristics : rapid growth, large size, necrosis, and the involvement of adjacent tissue3,6). In addition, up to 7% of both malignant and benign cases recur after incomplete resection4). Although it has been proposed that radiation therapy successfully stabilizes recurrent disease in intradural extramedullary tumors12), complete surgical resection is recommended as the initial treatment of choice. If a significant morbidity rate is anticipated, as with spinal cord tumors, adjuvant radiotherapy after tumorectomy may lead to better outcomes.
GrCT in the spinal cord is very rare. GrCT should be distinguished from other benign tumors such as schwannoma and meningioma because of its relatively high recurrence rate. Our cases and literature review suggest that the low signal intensity speckled dots may be a differential diagnostic point on MR images.
References
1. Buley ID, Gatter KC, Kelly PM, Heryet A, Millard PR. Granular cell tumours revisited. An immunohistological and ultrastructural study. Histopathology. 1988; 12:263–274. PMID: 2452781.

2. Critchley GR, Wallis NT, Cowie RA. Granular cell tumour of the spinal cord : case report. Br J Neurosurg. 1997; 11:452–454. PMID: 9474282.
3. Fanburg-Smith JC, Meis-Kindblom JM, Fante R, Kindblom LG. Malignant granular cell tumor of soft tissue : diagnostic criteria and clinicopathologic correlation. Am J Surg Pathol. 1998; 22:779–794. PMID: 9669341.
4. Kaiserling E, Ruck P, Xiao JC. Congenital epulis and granular cell tumor : a histologic and immunohistochemical study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995; 80:687–697. PMID: 8680977.
5. Markesbery WR, Duffy PE, Cowen D. Granular cell tumors of the central nervous system. J Neuropathol Exp Neurol. 1973; 32:92–109. PMID: 4346659.

6. Ordóñez NG, Mackay B. Granular cell tumor : a review of the pathology and histogenesis. Ultrastruct Pathol. 1999; 23:207–222. PMID: 10503740.
7. Pipeleers-Marichal M, Goossens A, De Waele B, KlÖppel G. Granular cell tumour of the appendix in a patient irradiated for a rectal carcinoma. Virchows Arch A Pathol Anat Histopathol. 1990; 417:177–180. PMID: 1695039.

8. Qu J, Ma J, Luo L, Ai L, Li S, Dai J. Subdural granular cell tumor in thoracic vertebral canal. Neurol India. 2009; 57:679–681. PMID: 19934580.

9. Rhee DJ, Choi YL, Suh YL, Park K. Atypical granular cell tumor of the sellar region. J Korean Neurosurg Soc. 2006; 40:459–462.
10. Strömblad LG, Brun A, Cameron R, Cronquist S. Spinal granular cell tumor with subarachnoid hemorrhage : case report. Neurosurgery. 1987; 21:230–233. PMID: 2821449.
11. Takayama Y, Hasuo K, Takahashi N, Nishimiya M, Nonoshita T, Takita Y, et al. Granular cell tumor presenting as an intradural extramedullary tumor. Clin Imaging. 2004; 28:271–273. PMID: 15246476.

12. Weinstein BJ, Arora T, Thompson LD. Intradural, extramedullary spinal cord granular cell tumor : a case report and clinicopathologic review of the literature. Neuropathology. 2010; 30:621–626. PMID: 20113407.

Fig. 1
Preoperative magnetic resonance (MR) images. A : Midsagittal T2-weighted fat-suppressed MR images show an isosignal intensity mass at T1-2. The spinal cord is displaced to the right posterior side. At the center of the tumor, there are low signal speckled dots. B and C : Mid-sagittal T1-weighted MR image (B) and gadolinium-enhanced fat-suppressed MR images (C) reveal isosignal intensity and a homogenous well-enhanced tumor. Speckled dots are observed at the center of the tumor. There is no dura tail sign. D : Axial MR image shows a well-circumscribed tumor displaced to the left side. It shows no extension to the foramen. E : Coronal MR image reveals that the tumor is located eccentrically in the intradural extramedullary and compresses the spinal cord.
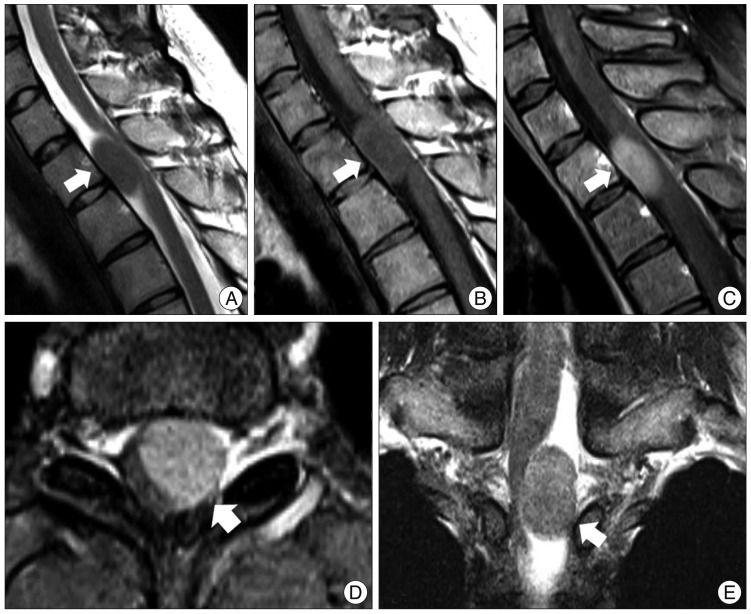
Fig. 2
Histologic examination. A and C : Cases 1 (A) and 2 (C) show granular cells with abundant eosinophilic granules in the cytoplasm. Hyalinizing fibrosis is also observed in all cases. Some lymphocytes have infiltrated the tumor. There is mild nuclear pleomorphism but no necrosis. B and D : The tumor cells are strongly and diffusely immunoreactive with S-100 protein in Case 1 (B) and Case 2 (D).
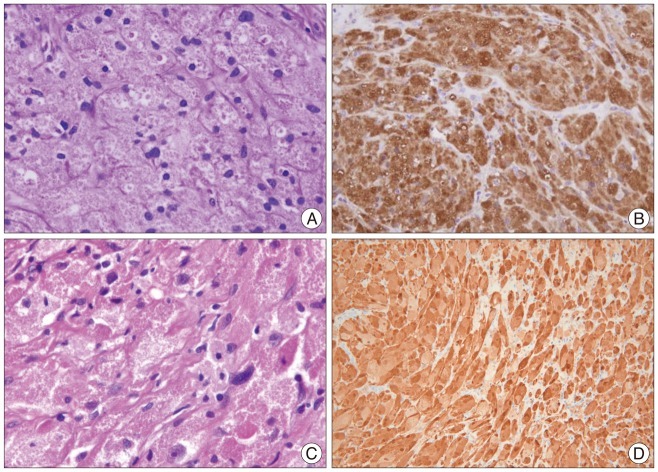
Fig. 3
Preoperative magnetic resonance (MR) images. A : A T2-weighted MR image shows an isosignal intensity mass at C5-6. B : A T2-sagittal MR image shows a tumor extending to the right foramen. Speckled dots in the center of the tumor are of low signal intensity in a T2-weighted MR image (arrow). C and D : T1-weighted MR images show an isointense and well-enhanced mass. E and F : Axial MR images show that the spinal cord is displaced to the left by the tumor. The tumor is mainly located in the extraforaminal area. Low signal speckled dots are observed in all sequences of MR images.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download