Abstract
Bilateral abducens nerve palsy related to ruptured aneurysm of the anterior communicating artery (ACoA) has only been reported in four patients. Three cases were treated by surgical clipping. No report has described the clinical course of the isolated bilateral abducens nerve palsy following ruptured ACoA aneurysm obliterated with coil. A 32-year-old man was transferred to our institution after three days of diplopia, dizziness and headache after the onset of a 5-minute generalized tonic-clonic seizure. Computed tomographic angiography revealed an aneurysm of the ACoA. Magnetic resonance imaging showed focal intraventricular hemorrhage without brain stem abnormalities including infarction or space-occupying lesion. Endovascular coil embolization was conducted to obliterate an aneurysmal sac followed by lumbar cerebrospinal fluid (CSF) drainage. Bilateral paresis of abducens nerve completely recovered 9 weeks after ictus. In conclusion, isolated bilateral abducens nerve palsy associated with ruptured ACoA aneurysm may be resolved successfully by coil embolization and lumbar CSF drainage without directly relieving cerebrospinal fluid pressure by opening Lillequist's membrane and prepontine cistern.
Sudden onset of bilateral abducens nerve palsy related to anterior communicating artery (ACoA) aneurysmal rupture is very rare. A review of literature disclosed only four patients in three case reports regarding isolated bilateral abducens nerve palsy following ruptured ACoA aneurysm2,5,10). Patients underwent surgical clipping of the aneurysms. The mechanisms of the paresis were speculated to be vasospasm of pontine branch of basilar artery5) or primary compression of the sixth-nerve caused by entrapment of cerebrospinal fluid (CSF) or blood clot2,10). The recovery period varied from post-operative day 3 to 3 months after onset.
To the best of our knowledge, we describe the first case of slow recovering bilateral abducens nerve palsy following endovascular coil embolization of ruptured ACoA aneurysm and discuss possible mechanisms.
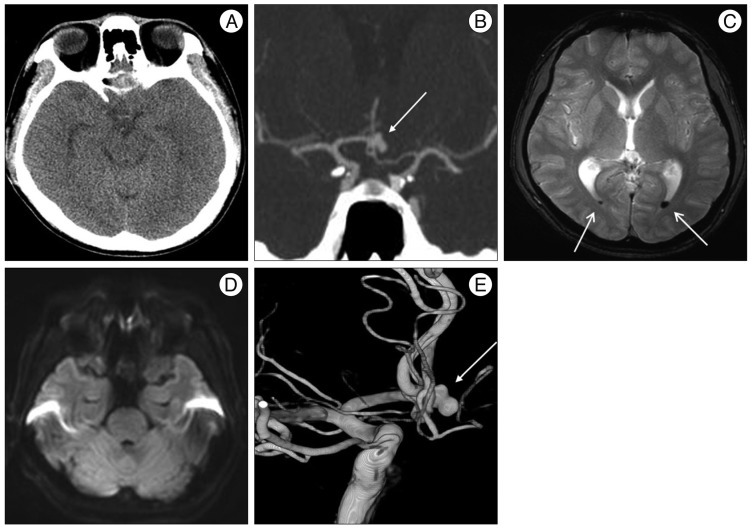
A 32-year-old man visited our institution for the evaluation of horizontal diplopia, dizziness and headache after the onset of a 5-minute generalized tonic-clonic seizure. On admission, the patient was alert and did not have any possible vascular risk factors such as hypertension, diabetes mellitus, hyperlipidemia, advanced age and cigarette smoking. The attending neurosurgeon and opthalomologist did not find any abnormality except slight nuchal rigidity and bilateral abducens nerve palsy (Fig. 2A). Initial cranial computed tomography (CT) (Fig. 1A) revealed no definite high density lesion in the brain including parenchyma, ventricle and cisternal space, but CT angiography (Fig. 1B) showed an aneurysm of the ACoA. Magnetic resonance imaging (MRI) (Fig. 1C, D) demonstrated focal intraventricular hemorrhage of the posterior horn of the lateral ventricle without abnormalities of the brain stem including infarction or space-occupying lesion. Catheter angiography was followed and a bilobulated saccular aneurysm (6.3×4.0 mm, dome×neck) without compression of brainstem was observed (Fig. 1E). Endovascular coil embolization was conducted to obliterate the aneurysmal sac without delay. Continuous lumbar drainage was performed for 6 days. A photograph taken 12 days later illustrated the improvement in both abducens nerve palsy (Fig. 2B). The signs of diplopia were resolved completely 9 weeks later (Fig. 2C). There were no clinical and radiologic signs of vasospasm during hospitalization.
Aneurysm is a rare etiology of isolated abducens nerve palsy and contributes to 2-3.6% of the incidence6-8). Only a few cases of abducens nerve palsy due to ruptured ACoA aneurysm have been reported.
Several possible mechanisms have been suggested. These include direct aneurysmal mass effect on abducens nerve, increased intracranial pressure induced by brain swelling or parenchyma hemorrhage, vasospastic pontine branch of basilar artery supplying to abducens nuclei5) and compression by the localization of CSF or hematoma in the cisternal space, particularly, in the prepontine cistern2,9,10).
Considering the size, location and direction of the ACoA aneurysm, the chance of direct aneurysmal compression on abducens nerve could be ruled out in this patient. Absence of marked swelling, acute hydrocephalus nor parenchyma hematoma was seen at initial CT, making the possibility of those etiologies low.
With regard to the possible explanation with vascular insufficiency, bilateral abducens nerve palsy by compromised abducens nuclei is more likely to accompany other focal lesion involving facial nuclei or medial longitudinal fasciculus because of their pertinent anatomical location1). MRI showed no diffusion restriction and space-occupying lesion on the brain stem. In addition, no evidence supported vasospasm. Thus, vasospasm could be excluded for the bilateral abducens nerve palsy in this patient. Posterior reversible encephalopathy syndrome (PRES) also can cause abducens nerve palsy and seizure3). But, the patient had no risk factors of PRES such as HTN, chronic liver failure, or organ transplantation. In addition, MRI revealed no signal change in the cortico-subcortical area of occipital and parietal lobes. Thus, PRES can be ruled out in this case.
Because a ruptured ACoA aneurysm can induce a profound clot in the basal cistern, primary brain stem compression by cisternal hematoma and secondary effect by acute hydrocephalus should be monitored closely5). In particular, the abducens nerve courses through the prepontine cistern, so a direct mass effect by CSF entrapment or clot can cause isolated bilateral abducens nerve palsy after ruptured ACoA aneurysm. Although, no prominent prepontine cistern and substantial cisternal change in the subsequent MRI was observed, we may speculate local CSF entrapment as the most probable mechanism for isolated bilateral abducens nerve palsy in this patient.
Lumbar CSF drainage was maintained for 6 days to prevent CSF accumulation and controlling headache refractory to medical treatment4). After lumbar drain insertion, headache was relieved. No supporting data about the effect of lumbar drainage for the treatment of abducens nerve palsy were found in previous articles. However, the possibility of reducing the CSF accumulation may exist. Therefore, we suspect that external drainage of CSF may lessen the compression effect of abducens nerve by CSF entrapment.
The recovery period of isolated abducens nerve palsy related to ruptured ACoA aneurysm has reportedly varied. Ziyal et al.10) and Göksu et al.2) described relatively early full recovery of abducens nerve palsy (3 days and 1 month after clipping, respectively). On the other hand, Nathal et al.5) reported a 3-month recovery time and we experienced a 9-week recovery interval. The difference may be explained by a different pathophysiology of the nerve paresis and treatment modality. Even though coil embolization cannot allow us to open prepontine cistern and Liliequist's membrane, isolated bilateral abducens nerve paralysis was fully recovered by 9 weeks from onset.
References
1. Brazis PW. Isolated palsies of cranial nerves III, IV, and VI. Semin Neurol. 2009; 29:14–28. PMID: 19214929.

2. Göksu E, Akyüz M, Gürkanlar D, Tuncer R. Bilateral abducens nerve palsy following ruptured anterior communicating artery aneurysm : report of 2 cases. Neurocirugia (Astur). 2007; 18:420–422. PMID: 18008016.

3. Hashmi M, Siddiqi SA, Saleem F, Mustafa MS. Posterior reversible leukoencephalopathy. J Pak Med Assoc. 2007; 57:468–470. PMID: 18072644.
4. Murad A, Ghostine S, Colohan AR. Role of controlled lumbar CSF drainage for ICP control in aneurysmal SAH. Acta Neurochir Suppl. 2011; 110(Pt 2):183–187. PMID: 21125469.

5. Nathal E, Yasui N, Suzuki A, Hadeishi H. Ruptured anterior communicating artery aneurysm causing bilateral abducens nerve paralyses--case report. Neurol Med Chir (Tokyo). 1992; 32:17–20. PMID: 1375980.

6. Patel SV, Mutyala S, Leske DA, Hodge DO, Holmes JM. Incidence, associations, and evaluation of sixth nerve palsy using a population-based method. Ophthalmology. 2004; 111:369–375. PMID: 15019392.

7. Rucker CW. The causes of paralysis of the third, fourth and sixth cranial nerves. Am J Ophthalmol. 1966; 61(5 Pt 2):1293–1298. PMID: 5938012.

8. Rush JA, Younge BR. Paralysis of cranial nerves III, IV, and VI. Cause and prognosis in 1,000 cases. Arch Ophthalmol. 1981; 99:76–79. PMID: 7458744.
9. Schneck MJ, Smith R, Moster M. Isolated bilateral abducens nerve palsy associated with traumatic prepontine hematoma. Semin Ophthalmol. 2007; 22:21–24. PMID: 17366113.

10. Ziyal IM, Ozcan OE, Deniz E, Bozkurt G, Ismailoğlu O. Early improvement of bilateral abducens nerve palsies following surgery of an anterior communicating artery aneurysm. Acta Neurochir (Wien). 2003; 145:159–161. discussion 161. PMID: 12601466.

Fig. 1
Initial CT shows no definite high density lesion in the basal cisterns (A), but CTA reveals an aneurysm of ACoA (white arrow) (B). Axial T2WI MR reveals a focal low signal (white arrows) of the posterior horn of the lateral ventricle which may represent intraventricular hemorrhage (C) without diffusion restriction of the brain stem (D). Three-dimensional-reconstructed digital subtraction angiography image demonstrates a bilobulated saccular aneurysm of ACoA (white arrow) (E). ACoA : anterior communicating artery.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download